Abstract
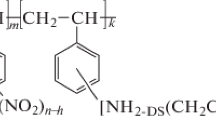
Two types of chelating sorbents with different types of addition of iminodipropionate groups to a polymeric matrix were synthesized: carboxyethylated aminopolystyrene (sorbent 1) based on linear polystyrene and carboxyethylaminomethylpolystyrene (sorbent 2) based on the copolymer of styrene and divinylbenzene. The ionization constants and concentrations of functional groups of the sorbents (exchange capacity for hydrogen ions) were determined. The sorbents exhibit high selectivity for copper(II) ions with the maximum of sorption from ammonia—acetate buffer solutions lying in a range of pH 5.0–7.5. The time needed for a solution of copper(II)—sorbent system with continuous stirring to reach exchange equilibrium is 3.5 and 2 h for sorbents 1 and 2, respectively. The exchange capacity for copper(II) ions is 2.54 and 0.10 mmol g−1, respectively.
Similar content being viewed by others
References
G. V. Myasoedova and S. B. Savvin, Khelatoobrazuyushchie sorbenty [Chelating Sorbents], Nauka, Moscow, 1984, 173 pp. (in Russian).
W. Rieman and H. Walton, Ion Exchange in Analytical Chemistry, Pergamon, New York, 1970.
M. Marhol, Ion Exchangers in Analytical Chemistry, Academia, Prague, 1982.
V. G. Sinyavskii, Selektivnye ionity [Selective Ion-Exchange Resins], Tekhnika, Kiev, 1967, 167 pp. (in Russian).
H. Leinonen and J. Lehto, React. Polym., 2000, 43, 1.
N. N. Matorina, L. V. Shepetyuk, L. V. Karlina, K. V. Chmutov, and N. V. Galitskaya, Zh. Fiz. Khim., 1978, 32, 1709 [Russ. J. Phys. Chem., 1978, 32, 1700 (Engl. Transl.)].
A. A. Luré, Sorbenty i khromatograficheskie materialy. Spravochnik [Sorbents and Chromatographic Materials. Reference Book], Khimiya, Moscow, 1979, 320 pp. (in Russian).
K. M. Saldadze and V. D. Kopylova-Valova, Komplekso-obrazuyushchie ionity [Complexation Ion-Exchange Resins], Khimiya, Moscow, 1980, 335 pp. (in Russian).
A. A. Vshivkov, L. K. Neudachina, and V. P. Melkozerov, in Khimiya (Programma “Universitety Rossii"”) [Chemistry (Program “University of Russia”)], Mosk. Gos. Univ., Moscow, 1994, 96 (in Russian).
D. Braun, Khimiya i tekhnologiya polimerov [Chemistry and Technology of Polymers], 1961, Issue 6, 66 (Russ. Transl.).
M. Kucharski, Polymery, 1966, 11, 251.
H. Zenftman, J. Chem. Soc., 1950, 982.
V. G. Sinyavskii, M. Ya. Romankevich, and N. P. Tsygankova, in Metody polucheniya khimicheskikh reaktivov i preparatov [Methods for Preparation of Chemical Reagents], IREA, Moscow, 1964, Issue 9, 17 (in Russian).
R. V. Davies, J. Kennedy, E. S. Lane, and J. L. Willans, J. Appl. Chem., 1959, 9, 368.
G. B. Bachman, H. Hellmar, K. R. Robinson, R. W. Finholt, E. J. Kahler, L. J. Fillar, L. V. Heisey, L. L. Lewis, and D. D. Micucci, J. Org. Chem., 1947, 12, 108.
H. Seliger, Makromol. Chem., 1973, 169, 83.
US Pat. 2195974; Chem. Abstrs, 1940, 34, 50937.
GB Pat. 478304; Chem. Abstrs, 1938, 32, 46101.
E. B. Trostyanskaya and G. Z. Nefedova, Zh. Anal. Khim., 1962, 17, 411 [J. Anal. Chem. USSR, 1962, 17, 400 (Engl. Transl.)].
Author’s Certificate 608799 USSR; Byul. izobret. [Invention Bulletin}], 1978, 20 (in Russian)
Author’s Certificate 645358 USSR; Byul. izobret. [Invention Bulletin}], 1981, 26 (in Russian)
US Pat. 4055586; Chem. Abstrs, 1977, 87, 24212t.
US Pat. 3899472; Chem. Abstrs, 1975, 83, 114436r.
N. M. Dyatlova, V. Ya. Temkina, and I. D. Kolpakova, Kompleksony [Complexones], Khimiya, Moscow, 1970, 417 pp. (in Russian).
V. P. Melkozerov, L. K. Neudachina, and A. A. Vshivkov, Zh. Obshch. Khim., 1997, 67, 99 [Russ. J. Gen. Chem., 1997, 67, 92 (Engl. Transl.)].
Yu. A. Skorik, L. K. Neudachina, A. A. Vshivkov, Yu. G. Yatluk, and N. V. Gert, Zh. Fiz. Khim., 1999, 73, 2269 [Russ. J. Phys. Chem., 1999, 73, 2055 (Engl. Transl.)].
M. Rhasi, J. Desbrieres, A. Tolaimate, M. Rinaundo, P. Vottero, and A. Alagui, Polymer, 2002, 43, 1267.
P. N. Nesterenko, A. V. Pirogov, and O. A. Shpigun, Zav. labor. [Industrial Laboratory], 2003, 69, 10 (in Russian).
P. V. Korostelev, Prigotovlenie rastvorov dlya khimiko-analiticheskikh rabot [Preparation of Solutions for Chemical Analytical Works], Akad. Nauk USSR, Moscow, 1962, 311 pp. (in Russian).
A. V. Ivanov, V. N. Figurovskaya, and V. M. Ivanov, Vestn. MGU, Ser. 2. Khim., 1992, 33, 570 [Vestn. Mosk. Univ., Ser. Khim., 1992, 33 (Engl. Transl.)].
L. J. Bellamy, The Infra-Red Spectra of Complex Molecules, John Wiley and Sons, New York, 1963.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 800–806, May, 2006.
Rights and permissions
About this article
Cite this article
Neudachina, L.K., Yatluk, Y.G., Baranova, N.V. et al. Synthesis and physicochemical properties of chelating sorbents containing functional groups of N-aryl-3-aminopropionic acids. Russ Chem Bull 55, 828–834 (2006). https://doi.org/10.1007/s11172-006-0339-3
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0339-3