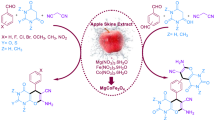
Abstract
In recent decades, the design of catalysts with features such as being readily recoverable from the reaction mixture, cost-effectiveness, efficiency, eco-friendly, and non-toxic is critical. Therefore, in this study, magnetic starch from apple seeds is suggested as a perfect substrate material with unique properties satisfying our need to minimize undesirable impacts on the environment. The magnetic starch acts as green support for cobalt nanoparticles to prepare Fe3O4@starch-Co(II) as an efficient heterogeneous catalyst in Mizoroki–Heck and Suzuki–Miyaura reactions. The fabricated catalyst was identified with several analysis techniques such as FT-IR, XRD, EDS, BET, TGA, FE-SEM, TEM, AAS, and elemental mapping. The catalyst performance reveals that it can be used as a promising replacement for palladium-based catalysts in the Mizoroki–Heck and Suzuki–Miyaura reactions. Because of the toxic nature of Pd-based materials, this catalyst can reduce the danger of using these catalysts. Also, due to the magnetic properties of the fabricated catalyst, the catalyst quickly separated from the reaction medium, and it is reusable for five runs without significant change in catalytic activity.
Graphical abstract














Similar content being viewed by others
Availability of data and materials
My manuscript has data included as electronic supplementary material.
Code availability
Not applicable.
Abbreviations
- AAS:
-
Atomic absorbtion spectroscopy
- BET:
-
Brunauer–Emmett–Teller analysis
- BJH:
-
Barrett–Joyner–Halenda analysis
- EDS:
-
Energy-dispersive spectroscopy
- FT-IR:
-
Fourier-transform infrared spectroscopy
- FE-SEM:
-
Field emission-scanning electron microscope
- GC:
-
Gas chromatography
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
- XRD:
-
X-ray diffraction analysis
- MCRs:
-
Multi-component reactions
- TLC:
-
Thin‐layer chromatography
References
A. Marandi, E. Nasiri, N. Koukabi, F. Seidi, Int. J. Biol. Macromol. 190, 61 (2021)
D. Khorsandi, A. Zarepour, I. Rezazadeh, M. Ghomi, R. Ghanbari, A. Zarrabi, F. T. Esfahani, N. Mojahed, M. Baghayeri, E. N. Zare, P. Makvandi (2022) Ionic liquid‐based materials for electrochemical biosensing. Clin. Transl. Discov. 2, e127 (2022).
R. Ghanbari, D. Khorsandi, A. Zarepour, M. Ghomi, A. Fahimipour, Z. Tavakkoliamol, and A. Zarrabi, Mater. Chem. Horizons (2022).
H. Mahdavi and R. Ghanbari, J. Ind. Eng. Chem. 113, 132 (2022).
T. Baran, N. Yılmaz Baran, A. Menteş, Appl. Organomet. Chem. 32, 4076 (2018)
S.M.S. Arabi, J. Alicata, D. Hanigan, S.R. Hiibel, Int. J. Greenh. Gas Control 111, 103472 (2021)
B. Rajarathinam, K. Kumaravel, G. Vasuki, RSC Adv. 6, 73848 (2016)
A. Marandi, N. Koukabi, M.A. Zolfigol, Res. Chem. Intermed. 47, 3145 (2021)
A. Marandi, E. Kolvari, M. Gilandoust, M.A. Zolfigol, Diam. Relat. Mater. 124, 108908 (2022)
P. Verma, S. Pal, S. Chauhan, A. Mishra, I. Sinha, S. Singh, V. Srivastava, J. Mol. Struct. 1203, 127410 (2020)
S. Sabaqian, F. Nemati, H.T. Nahzomi, M.M. Heravi, Carbohydr. Polym. 177, 165 (2017)
N. Shafiei, M. Nasrollahzadeh, T. Baran, N.Y. Baran, M. Shokouhimehr, Carbohydr. Polym. 262, 117920 (2021)
S. Zolfagharinia, N. Koukabi, E. Kolvari, RSC Adv. 6, 113844 (2016)
E. Kolvari, S. Zolfagharinia, RSC Adv. 6, 93963 (2016)
M.A. Khalilzadeh, S.Y. Kim, H.W. Jang, R. Luque, R.S. Varma, R.A. Venditti, M. Shokouhimehr, Mater. Today Chem. 24, 100869 (2022)
M. Arghan, N. Koukabi, E. Kolvari, Appl. Organomet. Chem. 33, e5075 (2019)
D. Yang, L. Yu, H. Chen, Y. Yu, Y. Xu, J. Sun, Y. Wang, Polym. Bull. 74, 5231 (2017)
A. Min Tong, W. Ya Lu, J. He Xu, G. Qiang Lin, Bioorg. Med. Chem. Lett. 14, 2095 (2004)
M. Manzoor, J. Singh, A. Gani, LWT 151, 112138 (2021)
T. Baran, J. Colloid Interface Sci. 496, 446 (2017)
A. Shaabani, A. Rahmati, Z. Badri, Catal. Commun. 9, 13 (2008)
H. Elkhenany, M. Abd Elkodous, N.I. Ghoneim, T.A. Ahmed, S.M. Ahmed, I.K. Mohamed, N. El-Badri, Int. J. Biol. Macromol. 143, 763 (2020)
A. Marandi, N. Koukabi, Colloids Surfaces A Physicochem. Eng. Asp. 621, 126597 (2021)
F. Chen, M. Huang, Y. Li, Ind. Eng. Chem. Res. 53, 8339 (2014)
T. Baran, A. Menteş, J. Organomet. Chem. 803, 30 (2016)
H.D. Güzel, M. Çalışkan, T. Baran, J. Phys. Chem. Solids 167, 110777 (2022)
K. Hong, M. Sajjadi, J.M. Suh, K. Zhang, M. Nasrollahzadeh, H.W. Jang, R.S. Varma, M. Shokouhimehr, A.C.S. Appl, Nano Mater. 3, 2070 (2020)
N. Khadir, G. Tavakoli, A. Assoud, M. Bagherzadeh, D.M. Boghaei, Inorganica Chim. Acta 440, 107 (2016)
T. Baran, I. Sargin, M. Kaya, A. Menteş, J. Mol. Catal. A Chem. 420, 216 (2016)
M. Nasrollahzadeh, R. Bakhshali-Dehkordi, T.A. Kamali, Y. Orooji, M. Shokouhimehr, J. Mol. Struct. 1244, 130873 (2021)
K.-H. Choi, M. Shokouhimehr, Y.-E. Sung, Bull. Korean Chem. Soc. 34, 1477 (2013)
Y. Wang, C. Lu, G. Yang, Z. Chen, J. Nie, React. Funct. Polym. 110, 38 (2017)
T. Baran, Int. J. Biol. Macromol. 127, 232 (2019)
A. Ahadi, S. Rostamnia, P. Panahi, L.D. Wilson, Q. Kong, Z. An, M. Shokouhimehr, Catal. 9, 140 (2019)
T. Baran, A. Menteş, J. Mol. Struct. 1122, 111 (2016)
T. Baran, İ Sargın, M. Kaya, P. Mulerčikas, S. Kazlauskaitė, A. Menteş, Chem. Eng. J. 331, 102 (2018)
M. Shokouhimehr, J.-H. Kim, Y.-S. Lee, Synlett 2006, 0618 (2006)
R.J. Kalbasi, F. Zamani, RSC Adv. 4, 7444 (2014)
A.R. Hajipour, F. Rezaei, Z. Khorsandi, Green Chem. 19, 1353 (2017)
A.R. Hajipour, Z. Khorsandi, Appl. Organomet. Chem. 34, e5398 (2020)
M. Arghan, N. Koukabi, E. Kolvari, Appl. Organomet. Chem. 32, e4346 (2018)
A.R. Hajipour, Z. Khorsandi, H. Karimi, Appl. Organomet. Chem. 29, 805 (2015)
Z. Zhu, J. Ma, L. Xu, L. Xu, H. Li, H. Li, ACS Catal. 2, 2119 (2012)
M. Arghan, N. Koukabi, E. Kolvari, Appl. Organomet. Chem. 33, e4823 (2019)
W. Affo, H. Ohmiya, T. Fujioka, Y. Ikeda, T. Nakamura, H. Yorimitsu, K. Oshima, Y. Imamura, T. Mizuta, K. Miyoshi, J. Am. Chem. Soc. 128, 8068 (2006)
A.R. Hajipour, Z. Khorsandi, Catal. Commun. 77, 1 (2016)
A.R. Hajipour, G. Azizi, Green Chem. 15, 1030 (2013)
H. Qi, W. Zhang, X. Wang, H. Li, J. Chen, K. Peng, M. Shao, Catal. Commun. 10, 1178 (2009)
P. Zhou, Y. Li, P. Sun, J. Zhou, and J. Bao, Chem. Commun. 43, 1418 (2007).
S. Iyer, V.V. Thakur, J. Mol. Catal. A Chem. 157, 275 (2000)
A.R. Hajipour, P. Abolfathi, Catal. Letters 147, 188 (2017)
K.S. Jithendra Kumara, G. Krishnamurthy, B.E. Kumara Swamy, N. Sunil-Kumar, M. Kumar, J. Porous Mater. 24, 1095 (2017)
M. Çalışkan, T. Baran, Appl. Clay Sci. 219, 106433 (2022)
M. Çalışkan, T. Baran, M. Nasrollahzadeh, J. Phys. Chem. Solids 152, 109968 (2021)
Acknowledgements
The authors sincerely acknowledge the Research Council of Semnan University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors are aware of the submission and agree to its publication, and have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koukabi, N., Arghan, M. Magnetic starch as green supports for cobalt nanoparticles: efficient, eco-friendly, and economical catalyst for Mizoroki–Heck and Suzuki–Miyaura reactions. Res Chem Intermed 48, 4553–4577 (2022). https://doi.org/10.1007/s11164-022-04818-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04818-2