Abstract
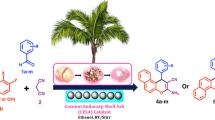
A simple and eco-friendly protocol for the facile synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyridine derivatives was developed using naturally sourced coconut endocarp shell ash (CESA) as a catalyst at room temperature in eco-compatible solvent system. The CESA catalyst was obtained from renewable feedstock, biodegradable waste, by simple thermal treatment on coconut endocarp shell (CESA), and the formation of its catalytic phase was characterized by DSC-TGA, FT-IR, XRD, EDX, BET, and SEM techniques. Derivatives of tetrahydrobenzo[b]pyran and 1, 4-dihydropyridine are obtained with an excellent yield, ranging from 82 to 99% in shorter reaction times. The remarkable advantages of the process presented here are that it is operationally clean, environmentally benign, and the product does not require chromatographic separation. Furthermore, the catalyst can be recovered conveniently and reused five times without significant loss of its catalytic activity.
Graphical abstract












Similar content being viewed by others
References
A. Yaghoubi, M.G. Dekamin, ChemistrySelect 2, 9236 (2017)
C. Altug, A.K. Burnett, E. Caner, Y. Durust, M.C. Elliott, R.P.J. Glanville, C. Guy, A.D. Westwell, Tetrahedron 67, 9522 (2011)
M. Nasr-Esfahani, T. Abdizadeh, J. Nanosci. Nanotechnol. 13, 5004 (2013)
S. Hatakeyama, N. Ochi, H. Numata, S. Takano, J. Chem. Soc. Chem. Commun. 17, 1202 (1988)
L.F. Tietze, Angew. Chem. Int. Ed. Engl. 22, 828 (1983)
A. Adili, Z.L. Tao, D.F. Chen, Z.Y. Han, Org. Biomol. Chem. 13(8), 2247 (2015)
S. Limsuwan, E.N. Trip, T.R.H.M. Kouwen, S. Piersma, A. Hiranrat, W. Mahabusarakam, S.P. Voravuthikunchai, J.M.V. Dijl, O. Kayser, Phytomedicine 16, 645 (2009)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
K. Görlitzer, A. Dehne, E. Engler, Arch. Pharm. 316, 264 (1983)
T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Bioorg. Med. Chem. Lett. 19, 1139 (2009)
Z.Q. Xu, K. Pupek, W.J. Suling, L. Enache, M.T. Flavin, Bioorg. Med. Chem. 14, 4610 (2006)
M. Brunavs, C.P. Dell, P.T. Gallagher, W.M. Owton, C.W. Smith, Eur. Pat. Appl. EP 557075 A1 19930825 (1993)
M. Rueping, E. Sugiono, E. Merino, Chem. Eur. J. 14, 6329 (2008)
Y. Gao, W. Yang, D.M. Du, Tetrahedron Asymmetry 23(5), 339 (2012)
S.K. Kundu, J. Mondal, A. Bhaumik, Dalton Trans. 42, 10515 (2013)
Y. Dgachi, L. Ismaili, D. Knez, M. Benchekroun, H. Martin, N. Szałaj, S. Wehle, O.M. Bautista-Aguilera, V. Luzet, A. Bonnet, B. Malawska, S. Gobec, M. Chioua, M. Decker, F. Chabchoub, J. Marco-Contelles, ChemMedChem 11, 1318 (2016)
G.A. Reynolds, K.H. Drexhage, Opt. Commun. 13, 222 (1975)
E.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, F.M.A. EI-Taweel, Heterocycles 26 903 (1987)
G. P. Ellis, in The Chemistry of Heterocyclic of Compounds. Chromenes, Harmones and Chromones; ed. By A. Weissberger, E.C. Taylor, (John Wiley: New York, 1977) 11
H. Langhals, Angew. Chem. Int. Ed. 43, 5290 (2004)
E.R. Bissell, A.R. Mitchell, R.E. Smith, J. Org. Chem. 45, 2283 (1980)
C.G. Knight, T. Stephens, Biochem. J. 258, 683 (1989)
M.G. Dekamin, M. Eslami, Green Chem. 16(12), 4914 (2014)
B. Karmakar, R. Nandi, Res. Chem. Intermed. 47, 2161 (2021)
S. Pradhan, V. Sahu, B.G. Mishra, J. Mol. Catal. A Chem. 425, 297 (2016)
D. Tahmassebi, J.A. Bryson, S.I. Binz, Synth. Commun. 41, 2701 (2011)
D.M. Pore, K.A. Undale, B.B. Dongare, U.V. Desai, Catal. Lett. 132, 104 (2009)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
S.N. Maddila, S. Maddila, W.E. Van-Zyl, S.B. Jonnalagadda, ChemistryOpen 5, 38 (2016)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
R. Ramesh, P. Vadivel, S. Maheswari, A. Lalitha, Res. Chem. Intermed. 42, 7625 (2016)
W.B. Sun, P. Zhang, J. Fan, S.H. Chen, Z.H. Zhang, Synth. Commun. 40, 587 (2010)
R. Rahnamafa, L. Moradi, M. Khoobi, Res. Chem. Intermed. 46(4), 2109 (2020)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
D. Azarifar, R. Nejat-Yami, F. Sameri, Z. Akrami, Lett. Org. Chem. 9, 435 (2012)
H. Zhi, C. Lu, Q. Zhang, J. Luo, Chem. Commun. 20, 2878 (2009)
V.U. Mane, S.M. Chavan, B.R. Choudhari, D.V. Mane, J. Pharm. Chem. Biol. Sci. 6(4), 311 (2019)
T. Tamoradi, B. Karmakar, M. Kamalzare, M. Bayat, A.T. Kal-Koshvndi, A. Maleki, J. Mol. Struct. 1219, 128598 (2020)
H. Malekim, J. Rakhtshah, B. Shaabani, Appl. Organomet. Chem. 34(8), e5683 (2020)
B. Loev, M.M. Goodman, K.M. Snader, R. Tedeschi, E. Macko, J. Med. Chem. 17, 956 (1974)
D.J. Triggle, Eur. J. Pharmacol. 375, 311 (1999)
R. Mannhold, B. Jablonka, W. Voigdt, K. Schonafinger, K. Schraven, Eur. J. Med. Chem. 27, 229 (1992)
S. Ghosh, F. Saikh, J. Das, A.K. Pramanik, Tetrahedron Lett. 54, 58 (2013)
R.S. Thombal, V.H. Jadhav, J. Chem. Applied Biochem. 2(1), 111 (2015)
A. Sakamoto, S.T. Ohnishi, R. Ogawa, J. Anaesth. 7(2), 193 (1993)
J.S. Yadav, B.V.S. Reddy, A.K. Basak, A.V. Narsaiah, Green Chem. 5, 60 (2003)
B. Sadeghi, A. Namakkoubi, A. Hassanabadi, J. Chem. Res. 37(1), 11 (2013)
X.Y. Wu, Synth. Commun. 42, 454 (2012)
K.L. Brigwood, E.G. Veitch, S.V. Ley, Org. Lett. 10(16), 3627 (2008)
A. Debache, W. Ghalem, R. Boulcina, A. Belfaitah, S. Rhouatiand, B. Carboni, Tetrahedron Lett. 50, 5248 (2009)
M. Safaiee, B. Ebrahimghasri, M.A. Zolfigol, S. Baghery, A. Khoshnood, D.A. Alonso, New J. Chem. 42, 12539 (2018)
M.A. Chari, K. Syamasundar, Catal. Commun. 6, 624 (2005)
M. Maheswara, V. Siddaiah, Y.K. Rao, Y.-M. Tzeng, C. Sridhar, J. Mol. Catal. A Chem. 260, 179 (2006)
G. Sabitha, G. Reddy, C.S. Reddy, J.S. Yadav, Tetrahedron Lett. 44, 4129 (2003)
S.D. Sharma, P. Hazarika, D. Konwar, Catal. Commun. 9, 709 (2008)
P. Sharma, M. Gupta, Green Chem. 2015(17), 1100 (2015)
S.T. Morbale, S.S. Shinde, S.D. Jadhav, M.B. Deshmukh, S.S. Patil, Der. Pharm. Lett. 7(12), 169 (2015)
A. Keyhani, F. Hatamjafari, Orient. J. Chem. 29(2), 783 (2013)
K. Venkateswarlu, Env. Chem. Lett. 19, 3887 (2021)
J. Song, B. Zhou, H. Zhou, L. Wu, Q. Meng, Z. Liu, B. Han, Angew. Chem. Int. Ed. 2015(54), 9399 (2015)
M. Satheesh, M. Pugazhvadivu, B. Prabu, V. Gunasegaran, A. Manikandan, J. Nanosci. Nanotechnol. 19, 4123 (2019)
T.L. Ting, R.P. Jaya, N.A. Hassan, H. Yaacob, D.S. Jayanti, M.A.M. Ariffine, J. Teknologi. 78, 85 (2016)
A. Apasi, P.B. Madakson, D.S. Yawas, V.S. Aigbodion, Tribo in Industry 34, 36 (2012)
M.U. Patil, S.K. Shinde, S.P. Patil, S.S. Patil, Res. Chem. Intermed. 46, 4923 (2020)
S.K. Shinde, S.A. Damate, S.T. Morbale, M.U. Patil, S.S. Patil, RSC Adv. 7, 7315 (2017)
S.K. Shinde, M.U. Patil, S.A. Damate, S.S. Patil, Res. Chem. sIntermed. 44(3), 1775 (2018)
A. Pawar, S. Gajare, A. Jagdale, S. Patil, W. Chandane, G. Rashinkar, S. Patil, Catal. Lett. 152, 1854 (2021)
Acknowledgements
The authors are grateful to the Indian Institute of Chemical Technology (IICT), Hyderabad and Common Facility Center (CFC), Shivaji University Kolhapur for spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, S.P., Shinde, S.K., Patil, M.U. et al. Coconut endocarp shell ash (CESA): a versatile and waste-originated catalyst for the synthesis of tetrahydrobenzo[b]pyrans and 1, 4-dihydropyridines. Res Chem Intermed 48, 3589–3612 (2022). https://doi.org/10.1007/s11164-022-04770-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04770-1