Abstract
In this work, the immobilized polythiophene on magnetic carbon nanotube (CNT–Fe3O4–PTh) was synthesized and introduced as a novel and recoverable catalytic system. The prepared magnetic heterogeneous nanocatalyst was characterized by FT-IR, TGA, EDX, VSM, XRD, TEM, and FE-SEM. Next, the catalytic efficiency of CNT–Fe3O4–PTh was evaluated through the green synthesis of a variety of dihydropyrimidinone and octahydroquinazolinone through Biginelli reaction between aromatic aldehydes, urea/thiourea, and β-dicarbonyl compounds under solvent-free conditions. The catalyst was magnetically recovered and recycled for five cycles without a discernible loss in its catalytic activity. Simple workup, affordability, short reaction times, mild reaction conditions, and the high yield of products are the interesting features of this project.
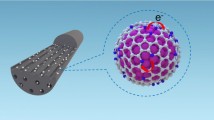
Graphic abstract
An environmentally benign nanocomposite was prepared using carbon nanotube as an inexpensive, nontoxic, and stable support in the preparation of CNT–Fe3O4–PTh, which was efficiently used as catalyst for the green synthesis of dihydropyrimidinone and octahydroquinazolinone derivatives through Biginelli reaction under solvent-free conditions.













Similar content being viewed by others
References
G. Kour, M. Gupta, B. Vishwanathan, K. Thirunavukkarasu, New J. Chem. 40, 8535 (2016)
Y. Zhai, Y. Dou, D. Zhao, P.F. Fulvio, R.T. Mayes, S. Dai, Adv. Mater. 23, 4828 (2011)
M.F. De Volde, S.H. Tawfick, R.H. Baughman, A.J. Hart, Science 339, 535 (2013)
A.P. Tsapenko, A.E. Goldt, E. Shulga, Z.I. Popov, K.I. Maslakov, A.S. Anisimov, P.B. Sorokin, A.G. Nasibulin, Carbon 130, 448 (2018)
P. Qi, O. Vermesh, M. Grecu, A. Javey, Q. Wang, H. Dai, S. Peng, K. Cho, Nano Lett. 3, 347 (2003)
A.B. Dalton, S. Collins, E. Munoz, J.M. Razal, V.H. Ebron, J.P. Ferraris, J.N. Coleman, B.G. Kim, R.H. Baughman, Nature 423, 703 (2003)
Q. Zhang, J.Q. Huang, W.Z. Qian, Y.Y. Zhang, F. Wei, Small 9, 1237 (2013)
E. Bertolucci, R. Bacsa, A. Benyounes, A.M. Raspolli-Galletti, M.R. Axet, P. Serp, ChemCatChem 7, 2971 (2015)
M. Bagherzadeh, A. Mortazavi-Manesh, RSC Adv. 6, 41551 (2016)
C.Ó. Dálaigh, SA Corr, Y. Gun'ko, SJ Connon, Angew. Chem. Int. Ed. 46, 4329 (2007)
SB Kalidindi, BR Jagirdar, Chemsuschem 5, 65 (2012)
C.W. Lim, I.S. Lee, Nano Today 5, 412 (2010)
A.D. Carswell, E.A. O’Rea, B.P. Grady, J. Am. Chem. Soc. 125, 14793 (2003)
Y. Sakurai, HS Jung, T. Shimanouchi, T. Inoguchi, S. Morita, R. Kuboi, K. Natsukawa, Sensors Actuators B Chem. 83, 270 (2002)
C. Born, R Pegn BJ Saiki, SK, sorry, Polym. Int. 63, 2061 (2014)
GR Chaudhary, P. Bansal, SK Mehta, Chem. Eng. J. 243, 217 (2014)
G. Lauro, M. Strocchia, S. Terracciano, I. Bruno, K. Fischer, C. Pergola, G. Bifulco, Eur. J. Med. Chem. 80, 407 (2014)
RK Yadlapalli, OP Chourasia, K. Vemuri, M. Sritharan, RS Perali, Bioorg. Med. Chem. Lett. 22, 2708 (2012)
IS Zorkun, S. Saraç, S. Çelebi, K. Erol, Bioorg. Med. Chem. 14, 8582 (2006)
N. Agarwal, P. Srivastava, SK Raghuwanshi, DN Upadhyay, S. Sinha, PK Shukla, VJ Ram, Bioorg. Med. Chem. 10, 869 (2002)
B.N. Naidu, M.E. Sorenson, M. Patel, Y. Ueda, J. Banville, F. Beaulieu, L. Pajor, Bioorg. Med. Chem. Lett. 25, 717 (2015)
B. Schnell, U.T. Strauss, P. Verdino, K. Faber, C.O. Kappe, Chem Inform 31, 32 (2000)
S. Jayakumar, TK Shabeer, J. Chem. Pharm. Res. 3, 1089 (2011)
S.A. Said, A.E.G.E. Amr, N.M. Sabry, M.M. Abdalla, Eur. J. Med. Chem. 44, 4787 (2009)
M.R. Bhosle, A.R. Deshmukh, S. Pal, A.K. Srivastava, R.A. Mane, Bioorg. Med. Chem. Lett. 25, 2442 (2015)
AR Trivedi, VR Bhuva, BH Dholariya, DK Dodiya, VB Kataria, VH Shah, Bioorg. Med. Chem. Lett. 20, 6100 (2010)
Z. Hassani, M.R. Islami, M. Kalantari, Bioorg. Med. Chem. Lett. 16, 4479 (2006)
H. Moghanian, M.A.B. Fard, A. Mobinikhaledi, N. Ahadi, Res. Chem. Intermed. 44, 4083 (2018)
G.D. Rao, B.N. Acharya, S.K. Verma, M.P. Kaushik, Tetrahedron Lett. 52, 809 (2011)
A. Rajack, K. Yuvaraju, C. Praveen, YLN Murthy, J. Mol. Catal. A Chem. 370, 197 (2013)
MG Chegini, M. Mokhtary, Polycycl. Aromat. Compd. 37, 63 (2017)
A. Debache, L. Chouguiat, R. Boulcina, B. Carbonib, Open Org. Chem. J. 6, 12 (2012)
F. Tamaddon, S. Moradi, J. Mol. Catal. A Chem. 370, 117 (2013)
J. Azuaje, CR Tubío, L. Escalante, M. Gómez, F. Guitián, A. Coelho, E. Sotelo, Appl. Catal. A Gen. 530, 203 (2017)
J. Mondal, T. Sen, A. Bhaumik, Dalton Trans. 41, 6173 (2012)
P. Akbarzadeh, N. Koukabi, E. Kolvari, Mol. Divers. 24, 319 (2020)
P. Akbarzadeh, N. Koukabi, E. Kolvari, Mol. Divers. (2019). https://doi.org/10.1007/s11030-019-10016-x
P. Akbarzadeh, N. Koukabi, Appl. Organomet. Chem. 34, e5395 (2020)
P. Akbarzadeh, N. Koukabi, Appl. Organomet. Chem. 34, e5746 (2020)
P. Akbarzadeh, N. Koukabi, E. Kolvari, Res. Chem. Intermed. 45(1009), 1–15 (2019)
P. Akbarzadeh, N. Koukabi, MM Hosseini, J. Heterocycl. Chem. 57, 2455 (2020)
M.A.E.A.A. Ali, A.M. Abu-Dief, Tetrahedron 71, 2579 (2015)
M.A.E. Aleem Ali El Remaily, A.M. Abu-Dief, R.M. El-Khatib, Appl. Organomet. Chem. 30, 1022 (2016)
M.A.E.A.A.A. El-Remaily, A.M. Abu-Dief, O. Elhady, Appl. Organomet. Chem. 33, e5005 (2019)
H. Tu, G. Wu, Y. Yi, M. Huang, R. Liu, X. Shi, H. Deng, Carbohydr. Polym. 210, 9 (2019)
A. Mehdinia, N. Khodaee, A. Jabbari, Anal. Chim. Acta 868, 1 (2015)
D.M. Lu, S.M. Yang, Synth. Met. 154, 73 (2005)
J. Li, S. Tang, L. Lu, H.C. Zeng, J. Am. Chem. Soc. 129, 9401 (2007)
A.M. Abu-Dief, I.F. Nassar, W.H. Elsayed, Appl. Organomet. Chem. 30, 917 (2016)
A.A. Marzouk, A.M. Abu-Dief, A.A. Abdelhamid, Appl. Organomet. Chem. 32, e3794 (2018)
S. Chidambaram, B. Pari, N. Kasi, S. Muthusamy, J. Alloys Compd. 665, 404 (2016)
M. Hemmati, M.M. Rajabi, A. Asghari, J. Chromatogr. A 1524, 1 (2017)
KR Gopinath, HB Premkumar, HS Shekar, KJ Rajendraprasad, H. Nagabhushana, K. Manjula, J. Pharm. Pharm. Sci. 5, 1579 (2016)
AS Paraskar, GK Dewkar, A. Sudalai, Tetrahedron Lett. 44, 3305 (2003)
Acknowledgements
The authors gratefully acknowledge the Research Council of the Semnan University for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akbarzadeh, P., Koukabi, N. Synthesis and characterization of the immobilized polythiophene on magnetic carbon nanotube as a prominent catalyst for the synthesis of dihydropyrimidinone and octahydroquinazolinone derivatives. Res Chem Intermed 46, 4955–4969 (2020). https://doi.org/10.1007/s11164-020-04234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04234-4