Abstract
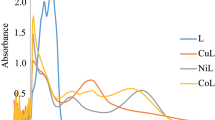
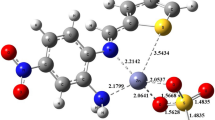
The ketoamine, 2-(1,3-benzothiazol-2-ylamino)naphthalene-1,4-dione(HL) and its Mn(II), Co(II), Ni(II), Cu(II), Pd(II) and Zn(II) complexes were synthesized and characterized by NMR, FTIR, and electronic spectroscopies with elemental, thermal, room temperature magnetic susceptibility, and molar conductivity measurements. The elemental, spectral, and thermal analysis corroborated the proposed molecular formulae for the complexes as [ML(OAc)]aH2O {M = Co, Ni, Cu, Zn} and [ML2]bH2O {M = Mn}. In addition, magnetic moments and electronic spectra data indicated a tetrahedral/square-planar geometry for all the metal complexes, while molar conductance measurements in DMSO established the covalent nature of the metal complexes. The in vitro antibacterial studies of the ketoamine and its metal complexes against K. oxytoca, P. aeruginosa, E. coli, B. cereus, and S. aureus showed that the compounds exhibited broad-spectrum antibacterial activities against the bacteria strains, with an inhibitory zone of 8.0–25.0 mm. Furthermore, molecular docking studies were conducted in order to examine the molecular interactions of the complexes with different enzymes of bacterial strains. The complexes showed good molecular interactions with all the receptors; phosphopentomutase, dioldehydratase, Ftsz, penicillin-binding protein 5, and peptidyl-tRNA hydrolase except for the bulky palladium complex. The results obtained from the molecular docking studies supported and provided a possible mechanistic basis for the experimentally observed antibacterial efficacies.






Similar content being viewed by others
References
N. Raman, A. Sakthivel, K. Rajasekaran, Synthesis and spectral characterization of antifungal sensitive schiff base transition metal complexes. Mycobiology 35, 150–153 (2007)
A.A. Al-Amiery, Inhibition effects of a synthesized novel 4-aminoantipyrine derivative on the corrosion of mild steel in hydrochloric acid solution together with quantum chemical studies. Med. Chem. Res. 21, 3204–3213 (2012)
D.S. Musmade, M.R. Sherkar, N.S. Pendbhaje, Synthesis and evaluation of some novel [3-isonicotinoyl-5-(4-substituted)-2,3-dihydro-1,3,4-oxadiazol-2-yl] derivatives for anti-inflammatory activity. Pharmacologyonline 1, 993–1000 (2011)
A. Ignatious, J. Rahul, P. Pushpa, R.T. Pardasani, Recent advances in 1,4 benzoquinone chemistry. J. Braz. Chem. Soc. 22, 385–421 (2011)
S. Patai, Z. Rappaport, The Chemistry of Quinonoid Compounds, vol. II (Wiley, New York, 1988)
R.H. Thomson, Naturally Occurring Quinones IV. Recent Advances (Blackie, London, 1997)
S. Spyros, Hydroxyquinones: synthesis and reactivity. Molecules 5, 1291–1330 (2000)
J. Yin, L.S. Liebeskind, Chemistry of trisquinones. J. Org. Chem. 63, 5726–5727 (1998)
I.L. Lluvia, D.N.F. Sendar, Y.S.B. Sonia, S.G. Aide, Naphthoquinones: biological properties and synthesis of lawsone and derivatives—a structured review. Rev. Fac. Quím. Farm. 21 (2014)
A.R. Mehendale, R.H. Thomson, Binaphthoquinones in lomatia ferruginea. Phytochemistry 14, 801–802 (1975)
U. Sharma, D. Katoch, S. Sood, N. Kumar, B. Singh, A. Thakur, Synthesis, antibacterial and antifungal activity of 2-amino-1,4-naphthoquinone using silica-supported perchloric acid (HClO4–SiO2) as a mild recyclable and highly efficient heterogeneous catalyst Gulati. Indian J. Chem. 52B, 1431–1440 (2013)
W. Nittayananta, K. Pangsomboon, P. Panichayupakaranant, N. Chanowanna, S. Chelae, V. Vuddhakul, P. Sukhumungoon, N. Pruphetkaew, Effects of lawsone methyl ether mouthwash on oral Candida in HIV-infected subjects and subjects with denture stomatisis. J. Oral Pathol. Med. 42, 698–704 (2013)
A. Mahapatra, T. Tshikalange, Synthesis and HIV-1 reverse transcriptase inhibition activity of 1,4-naphthoquinone derivatives. Chem. Nat. Compd. 47, 883–887 (2012)
G.J. Kapadia, G.S. Rao, R. Sridhar, E. Ichiishi, M. Takasaki, N. Suzuki, T. Konoshima, A. Iida, H. Tokuda, Chemoprevention of skin cancer: effect of Lawsonia inermis L. (Henna) leaf powder and its pigment artifact, lawsone in the Epstein–Barr virus early antigen activation assay and in two-stage mouse skin carcinogenesis models. Med. Chem. 13, 1500–1507 (2013)
S. Jiménez-Alonso, H.C. Orellana, A. Estévez-Braun, A.G. Ravelo, E. Pérez-Sacau, F. Machín, Design and synthesis of a novel series of pyranonaphthoquinones as topoisomerase II catalytic inhibitors. J. Med. Chem. 51, 6761–6772 (2008)
D. Bhasin, S.N. Chettiar, J.P. Etter, M. Mok, P.K. Li, Anticancer activity and SAR studies of substituted 1,4-naphthoquinones. Bioorg. Med. Chem. 21, 4662–4669 (2013)
F. Nourollah, V.P. Rahul, P.G. Shridhar, B.S. Fareed, G. Rajesh, Y.R. Sandhya, Crystal structure, NMR and theoretical investigations on 2-(o-hydroxy-anilino)-1,4-napthoquinone. J. Mol. Struct. 966, 144–151 (2010)
O. Pawar, A. Patekar, A. Khan, L. Kathawate, S. Haram, G. Markad, V. Puranik, S. Salunke-Gawali, J. Mol. Struct. 1059, 68–74 (2014)
S. Pal, M. Jadhav, T. Weyhermüller, Y. Patil, M. Nethaji, U. Kasabe, L. Kathawate, K.V. Badireenath, S. Salunke-Gawali, J. Mol. Struct. 1049, 355–361 (2013)
K. Laxmi, V.J. Pranya, K.D. Tapan, P. Sanjima, N. Milind, W. Thomas, G.P. Vedavati, V.K. Badireenath, S. Sunita, Reaction between lawsone and aminophenol derivatives: synthesis, characterization, molecular structures and antiproliferative activity. J. Mol. Struct. 1075, 397–405 (2014)
C. Bates, C. Pasternak, The incorporation of labeled amino sugars by Bacillus subtilis. Biochem. J. 96, 147–154 (1965)
H. Chmara, R. Andruszkiewicz, E. Borowski, Inactivation of glucosamine-6-phosphate synthetase from Salmonella typhimurium LT2 by fumaroyl diaminopropanoic acid derivatives, a novel group of glutamine analogs. BBA Protein Struct. Mol. Enzymol. 870, 357–366 (1986)
G. Jose, T.S. Kumara, G. Nagendrappa, H. Sowmya, J.P. Jasinski, S.P. Millikan, N. Chandrika, S.S. More, B. Harish, New polyfunctional imidazo[4,5-C]pyridine motifs: synthesis, crystal studies, docking studies and antimicrobial evaluation. Eur. J. Med. Chem. 77, 288–297 (2014)
P. Shyma, B. Kalluraya, S. Peethambar, S. Telkar, T. Arulmoli, Synthesis, characterization and molecular docking studies of some new 1,3,4-oxadiazolines bearing 6-methylpyridine moiety for antimicrobial property. Eur. J. Med. Chem. 68, 394–404 (2013)
A.A. Osowole, R. Kempe, R. Schobert, Synthesis, spectral, thermal, in vitro antibacterial and anticancer activities of some metal(II) complexes of 3-(-1-(4-methoxy-6-methyl)-2- pyrimidinylimino) methyl-2-napthol. Int. Res. J. Pure Appl. Chem. 2, 105–129 (2012)
A.A. Osowole, I. Ott, O.M. Ogunlana, Synthesis, spectroscopic, anticancer and antimicrobial properties of some metal(II) complexes of 2-[(2,3-dihydro-1H-inden-4-ylimino) methyl]-5-nitrophenol. Int. J. Inorg. Chem. 2012, 6 (2012)
A.A. Osowole, A.C. Ekennia, B.O. Achugbu, Synthesis, spectroscopic characterization and antibacterial properties of some metal(II) complexes of 2-(6-methoxybenzothiazol-2-ylimino)methyl)-4-nitrophenol. Res. Rev. J. Pharm. Anal. 2, 1–5 (2013)
A.A. Osowole, A.C. Ekennia, Synthesis, spectroscopic characterization and in vitro antibacterial properties of some metal(II) complexes of 4-nitro-6-[(4-thiophen-3-ylphenylimino)-methyl]-phenol. Cent. Point J. 19, 1–10 (2013)
G.A. Kolawole, A.A. Osowole, Synthesis and characterization of some metal(II) complexes of isomeric unsymmetrical Schiff-bases and their adducts with triphenyl phosphine. J. Coord. Chem. 62, 1437–1440 (2009)
A.C. Ekennia, D.C. Onwudiwe, A.A. Osowole, Synthesis, spectral, thermal stability and antibacterial studies of copper, nickel and cobalt complexes of N-methyl-N-phenyldithiocarbamate. J. Sulfur Chem. 36, 96–104 (2015)
A.C. Ekennia, D.C. Onwudiwe, C. Ume, E.E. Ebenso, Mixed ligand complexes of N-methyl-N-phenyl dithiocarbamate: synthesis, characterisation, antifungal activity, and solvent extraction studies of the ligand. Bioinorg. Chem. Appl. 2015, 10 (2015)
D.C. Onwudiwe, Y.B. Nthwane, A.C. Ekennia, E. Hosten, Synthesis, characterization and antimicrobial properties of some mixed ligand complexes of Zn(II) dithiocarbamate with different N-donor ligands. Inorg. Chim. Acta 447, 134–141 (2016)
W. Humphrey, A. Dalke, K. Schulten, VMD—visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996)
D. Santos-Martins, S. Forli, M.J. Ramos, A.J. Olson, AutoDock4(Zn): an improved AutoDock force field for small-molecule docking to zinc metalloproteins. J. Chem. Inf. Model. 54, 2371–2379 (2014)
O. Trott, A.J. Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461 (2010)
S.R. Cherayath, J. Alice, C.P. Prabhakaran, Palladium(II) complexes of Schiff bases derived from 5-amino-2,4-(1H,3H) pyrimidinedione (5-aminouracil) and 1,2-dihydro-1,5-dimethyl-2-phenyl-4-amino-3H-pyrazol-3-one. Trans. Met. Chem. 15, 449–453 (1990)
P.A. Ajibade, O.G. Idemudia, Synthesis, characterization and antibacterial studies of Pd(II) and Pt(II) complexes of some diaminopyrimidine derivatives. Bioinorg. Chem. Appl. 2013, 1–8 (2013)
T.L. Yang, W.W. Qin, Transition metal manganese(II), nickel(II), copper(II) and zinc(II)complexes of a new Schiff base ligand: synthesis, characterization and antitumor activity studies. Pol. J. Chem. 80, 1657–1662 (2006)
M.G. AbdEl-Wahed, M.S. Refat, M.S. El-Meghharbel, Metal complexes of antiuralethic drug: synthesis, spectroscopic characterization and thermal study on allopurinol complexes. J. Mol. Struct. 888, 416–429 (2008)
A.B.P. Lever, Inorganic Electronic Spectroscopy (Elsevier Publishers, Amsterdam, 1973)
N. Raman, S. Ravichandran, C. Thangaraja, Copper(II), cobalt(II), nickel(II) and zinc(II) complexes of Schiff base derived from benzil-2,4-dinitrophenylhydrazone with aniline. J. Chem. Sci. 116, 215–219 (2004)
M. Soenmez, M.A. Sekerci, New heterocyclic Schiff base and its metal complexes. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 34, 489–502 (2004)
M. Soenmez, A. Levent, M. Sekerci, Synthesis, characterization, and thermal investigation of some metal complexes containing polydentate ONOdonor heterocyclic Schiff base ligand. Russ. J. Coord. Chem. 30, 655–659 (2004)
D.C. Onwudiwe, P.A. Ajibade, Thermal Studies of Zn(II), Cd(II) and Hg(II) complexes of some N-alkyl-N-phenyl-dithiocarbamates. Int. J. Mol. Sci. 13, 9502–9513 (2012)
A.A. Osowole, R. Kempe, R. Schobert, K. Effenberger, Synthesis, spectroscopic, thermal, and in vitro anticancer properties of some M(II) complexes of 3-(-1-(4,6-dimethyl-2-pyrimidinylimino)methyl-2-naphthol. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 41, 825–833 (2011)
A.A. Osowole, S.A. Balogun, Spectral, magnetic, thermal and antibacterial properties of some metal(II) complexes of aminoindanyl schiff base. Eur. J. Appl. Sci. 4, 06–13 (2012)
P.L. Franceschini, M. Morstein, H. Berke, H.W. Schmalle, Volatile beta-ketoiminato- and beta-diketiminato-based zirconium complexes as potential MOCVD precursors. Inorg. Chem. 42, 7273–7282 (2003)
M.N. Patel, H.N. Joshi, C.R. Patel, Copper(II) complexes with norfloxacin and neutral terpyridines: cytotoxic, antibacterial, superoxide dismutase and DNA-interaction approach. Polyhedron 40, 159 (2012)
A.A. Abdel-Aziz, H.A. Elbadawy, Spectral, electrochemical, thermal, DNA binding ability, antioxidant and antibacterial studies of novel Ru(III) Schiff base complexes. Spectrochim. Acta 124, 404 (2014)
L. Malhota, S. Kumar, K.S. Dhindsa, Synthesis, characterization and microbial activity of Co(II), Ni(II), Cu(II) and Zn(II) complexes of aryloxyacetic acid and hydrazides. Indian J. Chem. 32A, 457–459 (1993)
D.P. Timothy, P.N. David, R.W. Guy, V.P. Vanessa, E.W. Brian, O.B. Brian, M.I. Tina, Bacillus cereus phosphopentomutase is an alkaline phosphatase family member that exhibits an altered entry point into the catalytic cycle. J. Biol. Chem. 286, 8043–8054 (2011)
N. Shibata, K. Mori, N. Hieda, Y. Higuchi, M. Yamanishi, T. Toraya, Release of a damaged cofactor from a coenzyme B12-dependent enzyme: X-ray structures of diol dehydratase-reactivating factor. Structure 13, 1745 (2005)
C.M. Tan, A.G. Therien, J. Lu, S.H. Lee, A. Caron, C.J. Gill, C. Lebeau-Jacob, L. Benton-Perdomo, J.M. Monteiro, P.M. Pereira, N.L. Elsen, J. Wu, K. Deschamps, M. Petcu, S. Wong, E. Daigneault, S. Kramer, L. Liang, E. Maxwell, D. Claveau, J. Vaillancourt, K. Skorey, J. Tam, H. Wang, T.C. Meredith, S. Sillaots, L. Wang-Jarantow, Y. Ramtohul, E. Langlois, F. Landry, J.C. Reid, G. Parthasarathy, S. Sharma, A. Baryshnikova, K.J. Lumb, M.G. Pinho, S.M. Soisson, T. Roemer, Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics. Sci. Transl. Med. 4, 635 (2012)
G. Nicola, J. Tomberg, R.F. Pratt, R.A. Nicholas, C. Davie, Crystal structure of Escherichia coli penicillin-binding protein 5 bound to a tripeptide boronic acid inhibitor: a role for Ser-110 in deacylation. Biochemistry 49, 8094 (2010)
A. Singh, A. Kumar, L. Gautam, P. Sharma, M. Sinha, A. Bhushan, P. Kaur, S. Sharma, A. Arora, T.P. Singh, Structural and binding studies of peptidyl-tRNA hydrolase from Pseudomonas aeruginosa provide a platform for the structure based inhibitor design against peptidyl-tRNA hydrolase. Biochem. J. 463, 329 (2014)
Acknowledgements
ACE thanks the University of Ibadan, Ibadan and Federal University, Ndufu-Alike, Ikwo, for the provision of research facilities and research leave, respectively. Dr. Damian C. Onwudiwe, North West University, South Africa is thanked for his assistance during characterization of synthesized compounds.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Osowole, A.A., Ekennia, A.C., Olubiyi, O.O. et al. Synthesis, spectral, thermal, antibacterial and molecular docking studies of some metal(II) complexes of 2-(1,3-benzothiazol-2-ylamino)naphthalene-1,4-dione. Res Chem Intermed 43, 2565–2585 (2017). https://doi.org/10.1007/s11164-016-2780-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2780-8