Abstract
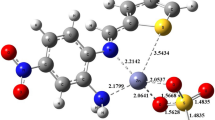
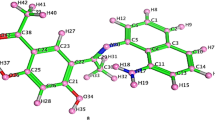
Schiff base ligand [3-(2-hydroxyphenylimino)-1,3-dihydroindol-2-one] was synthesized by the condensation reaction of isatin with 2-aminophenol. The Schiff base and its metal complexes with Co(II), Ni(II), and Cu(II) and ions were permeated by 1H NMR, IR, elemental analysis, ESI-MS spectroscopy, electronic spectroscopy, and some physicochemical measurements. The Schiff base behaved as a tridentate ligand in all metal complexes and was linked by azomethine nitrogen (=C=N). Supported by analytical data the metal-ligand stoichiometry in the formation of complexes was found as 1 : 2 molecular ratio. Based on analytical data allied with spectroscopic studies spilled that the Cu(II) complex preferred tetrahedral geometry, while Ni(II) and Co(II) complexes offered square planar and octahedral geometry, respectively. The complexes were undergone thermal analysis (TGA and DTG); complexes were found thermally stable up to 200°C. All the stable assembled compounds were assessed for antibacterial competency. The ligand and the complexes were played mild to sturdy antibacterial activity against numerous pathogenic bacterial species, although growth inhibitory activities of complexes were enhanced comparatively than their respective ligands. Additionally, molecular docking analysis and quantum computational calculations based on the density functional theory (DFT) approach were used to study the molecular characteristics of the novel complexes and provide in-depth insights into their involvement in their ability to restrict bacterial growth.








Similar content being viewed by others
REFERENCES
Mathur, G., Sharma, P.K., and Nain, S., Curr. Bioact. Mol., 2018, vol. 4, p. 211. https://doi.org/10.2174/1573407213666170221154354
Hossain, M.S., Zakaria, C.M., and Kudrat-E-Zahan, Md., Asian J. Res. Chem., 2017, vol. 10, p. 6. https://doi.org/10.5958/0974-4150.2017.00002.5
Sergienko, V.S., Koksharova, T.V., and Surazhskaya, M.D., Russ. J. Inorg. Chem., 2018, vol. 63, p. 1171. https://doi.org/10.1134/S0036023618090176
Pahontu, E., Julea, F., Rosu, T., Purcarea, V., Chumakov, Y., Petrenco, P., and Gulea, A., J. Cell. Mol. Med., 2015, vol. 19, p. 865. https://doi.org/10.1111/jcmm.12508
Pandeya, S.N., Sriram, D., Nath, G., and Declercq, E., Eur. J. Pharmacol., 1999, vol. 9, p. 25. https://doi.org/10.1016/s0928-0987(99)00038-x
Vinogradova, K.A., Rakhmanova, M.I., and Nikolaenkova, E.B., Russ. J. Coord. Chem., 2022, vol. 48, p. 301. https://doi.org/10.1134/S1070328422050098
Patel, P.N., Desai, D.H., and Patel, N.C., Russ. J. Coord. Chem., 2021, vol. 47, p. 909. https://doi.org/10.1134/S1070328421120010
Zhu, X., Wang, C., Lu, Z., and Dang, Y., Trans. Metal Chem., 1997, vol. 22, p. 9. https://doi.org/10.1023/A:1018453316348
Tian, X., Li, Y., and Zhang, Y., J. Struct. Chem., 2021, vol. 62, p.1872. https://doi.org/10.1134/S0022476621120076
Latif, M.A., Tofaz, T., Chaki, B.M., Tariqul Islam, H.M., Hossain, M.S., and Kudrat-E-Zahan, M., Russ. J. Gen. Chem., 2019, vol. 89, p.1197. https://doi.org/10.1134/S107036321906015X
Chohan, Z.H., Pervez, H., Rauf, A., Khan, K.M., and Supuran, C.T., J. Enzyme Inhib. Med. Chem., 2004, vol. 19, p. 417. https://doi.org/10.1080/14756360410001710383
Uvarova, M.A. and Nefedov, S.E., Russ. J. Inorg. Chem., 2021, vol. 66, p. 1837. https://doi.org/10.1134/S0036023621120202
Shaxma, C.L., Mishra, V., and Narvi, S.S., Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 2006, vol. 16, p. 243. https://doi.org/10.1080/00945718608057529
Nakamato, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1978.
West, D.X., Ooms, C.E., Saleda, J.S., Gebremedhin, H., and Liberta, A.E., Trans. Metal Chem., 1994, vol. 19, p. 553.
Farhana, A., Dalia, S.A., Hossain, M.S., Sarker, S., and Kudrat-E-Zahan, M., Asian J. Chem. Sci., 2018, vol. 4, p. 1. https://doi.org/10.9734/ajocs/2018/40913
Yambulatov, D.S., Nikolaevskii, S.A., and Lutsenko, I.A., Kiskin, M.A., Shmelev, M.A., Bekker, O.B., Efimov, N.N., Ugolkova, E.A., Minin, V.V., Sidorov, A.A., and Eremenko, I.L., Russ. J. Coord. Chem., 2020, vol. 46, p. 772. https://doi.org/10.1134/S1070328420110093H
Comprehensive Coordination Chemistry, ed. G. Wilkinson, Oxford: Pergamon press, 1987, vol. 6, p. 325.
The Chemistry of Functional Groups, Patai, S., Ed., New York: Wiley, 1983, p. 140.
Ismael, M., Abdou, A., and Abdel-Mawgoud, A.M., Z. anorg. allg. Chem., 2018, p. 1203. https://doi.org/10.1002/zaac.201800230
Abdou, A., Omran, O.A., Nafady, A., and Antipin, I.S., Arab. J. Chem., 2022, vol. 15, p. 103656. https://doi.org/10.1016/j.arabjc.2021.103656
Ismael, M., Abdel-Mawgoud, A.M., Rabia, M.K., and Abdou, A., J. Mol. Struct., 2021, vol. 1227, p. 129695. https://doi.org/10.1016/j.molstruc.2020.129695
Ismael, M., Abdel-Mawgoud, A.M., Rabia, M.K., and Abdou, A., J. Mol. Liq., 2021, vol. 330, p. 115611. https://doi.org/10.1016/j.molliq.2021.115611
Hashem, H.E., Nath, A., and Kumer, A., J. Mol. Struct., 2022, vol. 1250, p. 131915. https://doi.org/10.1016/j.molstruc.2021.131915
Ismael, M., Abdel-Mawgoud, A.M., Rabia, M.K., and Abdou, A., Inorg. Chim. Acta, 2020, vol. 505, p. 119443. https://doi.org/10.1016/j.ica.2020.119443
Abdou, A. and Mawgoud, M.A., Appl. Organomet. Chem., 2022, p. 6600. https://doi.org/10.1002/aoc.6600
Mohapatra, R.K., Sarangi, A.K., Azam, M., El-Ajaily, M.M., Kudrat-E-Zahan, M., Patjoshi, S.B., and Dash, D.C., J. Mol. Struct., 2019, vol. 1179, p. 65. https://doi.org/10.1016/j.molstruc.2018.10.070
Kudrat-E-Zahan, Md. and Islam, M.S., Russ. J. Gen. Chem., 2015, vol. 85, p. 979. https://doi.org/10.1134/S1070363215040350
Shokr, E.Kh., Kamel, M.S., Abdel-Ghany, H., El-Remaily, M.A.A.A., and Abdou, A., Mater. Chem. Phys., 2022, vol. 290, p. 126646. https://doi.org/10.1016/j.matchemphys.2022.126646
Elkanzi, N.A.A., Ali, A.M., Hrichi, H., and Abdou, A., Appl. Organomet. Chem., 2022, vol. 36, p. e6665. https://doi.org/10.1002/aoc.6665
Abdou, A., J. Mol. Struct., 2022, vol. 1262, p. 132911. https://doi.org/10.1016/j.molstruc.2022.132911
Abu-Dief, A.M., Alotaibi, N.H., Al-Farraj, E.S., Qasem, H.A., Alzahrani, S., Mahfouz, M.K., and Abdou, A., J. Mol. Liq., 2022, vol. 365, p. 119961. https://doi.org/10.1016/j.molliq.2022.119961
Hrichi, H., Elkanzi, N.A.A., Ali, A.M., and Abdou, A., Res. Chem. Intermed., 2022. https://doi.org/10.1007/s11164-022-04905-4
Abdou, A., Mostafa, H.M., and Abdel-Mawgoud, A.M., Inorg. Chim. Acta, 2022, vol. 539, p. 121043. https://doi.org/10.1016/j.ica.2022.121043
Abdou, A., Mostafa, H.M., and Abdel-Mawgoud, M.A., Sohag J. Sci., 2022, vol. 6, p. 167. https://doi.org/10.21608/SJSCI.2022.151396.1016
Elkanzi, N.A.A., Ali, A.M., Albqmi, M., and Abdou, A., Appl. Organomet. Chem., 2022, vol. 36, p. e6868. https://doi.org/10.1002/aoc.6868
Elkanzi, N.A.A., Hrichi, H., Salah, H., Albqmi, M., Ali, A.M., and Abdou, A., Polyhedron, 2023, vol. 230, p. 116219. https://doi.org/10.1016/j.poly.2022.116219
Alghuwainem, Y.A.A., Abd El-Lateef, H.M., Khalaf, M.M., Abdelhamid, A.A., Alfarsi, A., Gouda, M., Abdelbaset, M., and Abdou, A., J. Mol. Liq., 2023, vol. 369, p. 120936. https://doi.org/10.1016/j.molliq.2022.120936
Alghuwainem, Y.A.A., El-Lateef, H.M.A., Khalaf, M.M., Amer, A.A., Abdelhamid, A.A., Alzharani, A.A., Alfarsi, A., Shaaban, S., Gouda, M., and Abdou, A., Int. J. Mol. Sci., 2022, vol. 23, p. 15614. https://doi.org/10.3390/ijms232415614
Arafath, Md.A., Adam, F., Ahamed, M.B.K., Karim, M.R., Uddin, Md.N., Yamin, B.M., and Abdou, A., J. Mol. Struct., 2022, p. 134887. https://doi.org/10.1016/j.molstruc.2022.134887
ACKNOWLEDGMENTS
The authors are thankful to the Department of Chemistry, University of Rajshahi, Bangladesh for providing the laboratory facilities. The part of this work is supported by the UGC project funded by Begum Rokeya University, Rangpur, Bangladesh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Hossain, M.S., Khushy, K.A., Latif, M.A. et al. Co(II), Ni(II), and Cu(II) Complexes Containing Isatin-Based Schiff Base Ligand: Synthesis, Physicochemical Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis. Russ J Gen Chem 92, 2723–2733 (2022). https://doi.org/10.1134/S1070363222120222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222120222