Abstract
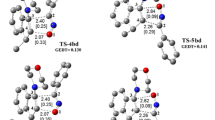
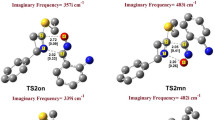
The 1,3-dipolar cycloadditions are extensively used for the preparation of five-membered heterocycles. A mechanism consisting of two pathways was proposed for production of two regioisomers of the 1,3-dipolar cycloaddition of the nitrilimine and a thione-containing dipolarophile. Here, we have investigated the kinetics and mechanism of this reaction using density functional theory. Two possible mechanisms of A and B have been investigated in which Cl− is present in the structure of the nitrilimine reactant in the case of mechanism A, while it is absent in the case of mechanism B. Mechanism A, involving Cl−, has higher barrier energy than mechanism B, and so is rejected. Mechanism B involves two pathways, I and II, which lead to two regioisomers with different percentages in the products. Both of the pathways are one-step. Pathway II involves the attack of the nitrogen atom of the nitrilimine on the carbon atom of the C=S group of the dipolarophile. The product of pathway II is kinetically and thermodynamically a more favorable product than its regioisomer produced in the other pathway. The obtained results are in agreement with the experimental results.















Similar content being viewed by others
References
R. Huisgen, M. Seidel, G. Wallbillich, H. Knupfer, Tetrahedron 17, 3 (1962)
R. Huisgen, Angew. Chem. Int. Ed. 2, 565 (1963)
R. Huisgen, W. Fliegl, W. Kolbeck, Chem. Ber. 116, 3027 (1983)
G. Molteni, A. Ponti, Tetrahedron Asymmetry 15, 3711 (2004)
G. Molteni, A. Ponti, Tetrahedron Asymmetry 19, 1381 (2008)
G. Molteni, Tetrahedron Asymmetry 15, 1077 (2004)
G. Molteni, Heterocycles 65, 2513 (2005)
A.S. Shawali, M.A. Abdallah, M.A.N. Mosselhi, M.S. Elewa, J. Heterocycl. Chem. 44, 285 (2007)
A. Agocs, A. Benyei, L. Somogyi, P. Herczegh, Tetrahedron Asymmetry 9, 3359 (1998)
G. Broggini, L. Garanti, G. Molteni, G. Zecchi, Heterocycles 53, 831 (2000)
H.N. Dogan, A. Duran, S. Rollas, G. Sener, M.K. Uysal, D. Gulen, Bioorg. Med. Chem. 10, 2893 (2002)
N. Solak, S. Rollas, Arkivoc xii, 173 (2006)
H.N. Hafez, M.I. Hegab, I.S.A. Farag, A.B.A. El-Gazzar, Bioorg. Med. Chem. Lett. 18, 4538 (2008)
A.R. Jalilian, S. Sattari, M. Bineshmarvasti, A. Shafiee, M. Daneshtalab, Arch. Pharm. Weinheim 333, 347 (2000)
A. Shafiee, M. Mohamadpour, F. Abtahi, A. Khoyi, J. Pharm. Sci. 70, 510 (1981)
M.A. Hosny, T.H. El Sayed, E.A. El Sawi, Eur. J. Chem. 9, 1276 (2012)
D. Guo, Z. Wang, Z. Fan, H. Zhao, W. Zhang, J. Cheng, J. Yang, Q. Wu, Y. Zhang, Q. Fan, Chin. J. Chem. 30, 2522 (2012)
E.W. Thomas, E.E. Nishizawa, D.C. Zimmermann, D.J. Williams, J. Med. Chem. 28, 442 (1985)
W. Benchouk, S.M. Mekelleche, Theochem 862, 1 (2008)
A. Ponti, G. Molteni, J. Org. Chem. 66, 5252 (2001)
F. Moeinpour, M. Bakavoli, A. Davoodnia, A. Morsali, J. Tetrahedron Comput. Chem. 11(1), 99 (2012)
F. Moeinpour, Chin. J. Chem. Phys. 23(2), 165 (2010)
L.R. Domingo, M.J. Aurell, M. Arno, J.A. Saez, J. Mol. Struct. Theochem 811, 125 (2007)
I. Zghab, B. Trimeche, D. Touboul, H.B. Jannet, C. R. Chim. 17, 171 (2014)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
M.J. Frisch et al., Gaussian 03, Revision C.02 (Gaussian Inc., Pittsburgh, PA, 2003)
J. Tomasi, R. Cammi, J. Comput. Chem. 16, 1449 (1995)
S.A. Beyramabadi, H. Eshtiagh-Hosseini, M.R. Housaindokht, A. Morsali, Organometallics 27, 72 (2008)
H. Wang, Res. Chem. Intermed. 38, 2175 (2012)
S.H. Vahidi, A. Morsali, S.A. Beyramabadi, Comput. Theor. Chem. 994, 41 (2012)
M. Najafi Ardabili, A. Morsali, S.A. Beyramabadi, H. Chegini, A. Gharib, Res. Chem. Intermed. 41, 5389 (2015)
H. Eshtiagh-Hosseini, S.A. Beyramabadi, A. Morsali, M. Mirzaei, H. Chegini, M. Elahi, M.A. Naseri, J. Mol. Struct. 1072, 187 (2014)
A.A. Altaf, A. Shahzad, Z. Gul, S.A. Khan, A. Badshah, M.N. Tahir, Z.I. Zafar, E. Khan, Eur. J. Chem. 1, 221 (2010)
H.S. Dong, B. Wang, J. Chem. Cryst. 35, 61 (2005)
R. Reyes-Martínez, R. Mejia-Huicochea, J.A. Guerrero-Alvarez, H. Höpfl, H. Tlahuext, ARKIVOC 19, 19–30 (2008)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bazian, A., Beyramabadi, S.A., Davoodnia, A. et al. Kinetics and mechanism of the 1,3-dipolar cycloaddition of nitrilimine with thione-containing dipolarophile: a detailed DFT study. Res Chem Intermed 42, 6125–6141 (2016). https://doi.org/10.1007/s11164-016-2449-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2449-3