Abstract
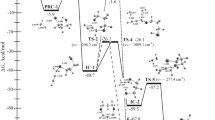
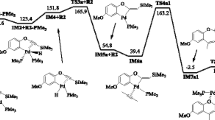
In this study the alkoxide-independent pathway of reductive elimination of C–O from palladium (p-cyanophenyl) neopentoxide complex in THF at 47 °C was investigated theoretically by use of density functional theory and the solvent polarized continuum model. On the basis of experimental results a two-pathway mechanism had been proposed: a concerted process (path a) and a two-step process (path b) initiated by inner-sphere attack of the alkoxide ligand on the ipso-carbon atom of the palladium-bound aryl group (step 1) then Pd–C heterolysis to form p-neopentoxybenzonitrile (step 2). The activation energies of the two pathways were calculated by use of the quantum mechanical approach and compared with each other and with experimental results.









Similar content being viewed by others
References
A. de Meijere, F. Diederich, Metal-Catalyzed Cross-Coupling Reactions (Wiley, Weinheim, 2004)
J. Tsuji, Palladium Reagents And Catalysts. New Perspectives for the 21st Century (Wiley, London, 2004)
S.A. Beyramabadi, H. Eshtiagh-Hosseini, M.R. Housaindokht, A. Morsali, Organometallics 27, 72 (2008)
S.A. Beyramabadi, H. Eshtiagh-Hosseini, M.R. Housaindokht, A. Morsali, J. Mol. Struct. (THEOCHEM) 903, 108 (2009)
H. Eshtiagh-Hosseini, S.A. Beyramabadi, A. Morsali, M.R. Housaindokht, J. Mol. Struct. (THEOCHEM) 941, 138 (2010)
L.J. Goossen, D. Koley, H.L. Hermann, W. Thiel, Organometallics 24, 2398 (2005)
E.-I. Negishi, A. de Meijere, Handbook of Organ Palladium Chemistry for Organic Synthesis (Wiley, New York, 2002)
N. Miyaura, A. Suzuki, Chem. Rev. 95, 2457 (1995)
A.B. Patel, K.H. Chikhalia, P. Kumari, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1377-8
A. De Meijere, F.E. Meyer, Chem. Int. Ed. Engl. 33, 2379 (1995)
I.P. Beletskaya, A.V. Cheprakov, Chem. Rev. 100, 3009 (2000)
J.K. Stille, Angew. Chem. Int. Ed. Engl. 25, 508 (1986)
Q. Du, Y. Li, Res. Chem. Intermed. 38, 1807 (2012)
J.P. Wolfe, H. Tomori, J.P. Sadighi, J. Yin, S.L. Buchwald, J. Org. Chem. 65, 1158 (2000)
R.A. Widenhoefer, H.A. Zhong, S.L. Buchwald, J. Am. Chem. Soc. 119, 6787 (1997)
R.A. Widenhoefer, S.L. Buchwald, J. Am. Chem. Soc. 120, 6504 (1998)
A.D. Becke, J. Chem. Phys. 98, 5648 (1993)
A.D. Becke, Phys. Rev. A 38, 3098 (1988)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery Jr, T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Gaussian 03 (Gaussian Inc., Pittsburgh, 2003)
P.J. Hay, W.R. Wadt, J. Chem. Phys. 82, 299 (1985)
S.H. Vahidi, A. Morsali, S.A. Beyramabadi, Comput. Theor. Chem. 994, 41 (2012)
A. Morsali, F. Hoseinzade, A. Akbari, S.A. Beyramabadi, R. Ghiasi, J. Solut. Chem. 42, 1902 (2013)
A. Akbari, F. Hoseinzade, A. Morsali, S.A. Beyramabadi, Inorg. Chim. Acta 394, 423 (2013)
S. Miertus, E. Scrocco, J. Tomasi, Chem. Phys. 55, 117 (1981)
J. Tomasi, M. Persico, Chem. Rev. 94, 2027 (1994)
R. Cammi, J. Tomasi, J. Comput. Chem. 16, 1449 (1995)
J.F. Hartwig, Inorg. Chem. 46, 1936 (2007)
Acknowledgments
We thank the Center of Theoretical Research of the Kharazmi Institute for allocation of computer time.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ardabili, M.N., Morsali, A., Beyramabadi, S.A. et al. Quantum mechanical study of the alkoxide-independent pathway of reductive elimination of C–O from palladium (p-cyanophenyl) neopentoxide complex. Res Chem Intermed 41, 5389–5398 (2015). https://doi.org/10.1007/s11164-014-1640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1640-7