Abstract
Climate change stressors (e.g., warming and ocean acidification) are an imminent challenge to the physiological performance of marine organisms. Several studies spanning the last decade have reported widespread effects of warming and acidification on marine fishes, especially teleosts, but more work is needed to elucidate the responses in marine elasmobranchs, i.e., sharks and rays. Dispersal capacity, as a result of locomotor performance, is a crucial trait that will determine which group of elasmobranchs will be more or less vulnerable to changes in the environment. In fact, efficient and high locomotor performance may determine the capacity for elasmobranchs to relocate to a more favorable area. In this review we integrate findings from work on locomotion of marine sharks and rays to identify characteristics that outline potential vulnerabilities and strength of sharks and rays under climate change. Traits such as intraspecific variability in response to climatic stressors, wide geographic range, thermotaxis, fast swimming or low energetic costs of locomotion are likely to enhance the capacity to disperse. Future studies may focus on understanding the interacting effect of climatic stressors on morphology, biomechanics and energetics of steady and unsteady swimming, across ontogeny and species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is considered one of the biggest challenges for governments and managers as it may significantly accelerate loss of biodiversity (Intergovernmental Panel on Climate Change 2018; Arneth et al. 2020; Manes et al. 2021). Anthropogenic activity has increased atmospheric greenhouse gases concentration, particularly carbon dioxide (pCO2), causing the oceans to become warmer and more acidic (Dupont and Pörtner 2013; Intergovernmental Panel on Climate Change 2018). Climate models project a rise in pCO2 to levels of about 1000 ppm by the year 2100 (Raven et al. 2005). As a consequence, the ocean pH is expected to decline by up to 0.4 units by the end of the century (Gattuso et al. 2010; Riebesell et al. 2011). Even though ocean warming and acidification are considered to be some of the biggest threats to marine biodiversity, the individual and synergistic effects of these stressors on elasmobranch fishes (sharks and rays) are still clearly understudied (Rosa et al. 2017a; Di Santo 2019; Wheeler et al. 2020; Bouyoucos et al. 2020). Previous work on ocean acidification and warming has focused mostly on teleost fishes while the effect on elasmobranchs has been investigated to a much lesser degree (for example: Lauder and Di Santo 2015; Johnson et al. 2016; Rosa et al. 2017a, b; Di Santo 2019; Wheeler et al. 2020; Mirasole et al. 2020).
Marine organisms vary in their physiological and morphological characteristics and, as a result, their individual responses to ocean warming and ocean acidification differ significantly (Harvey et al. 2013). For this reason, efforts may be focused on identifying key traits that make “physiotypes”, i.e., organisms with similar physiological characteristics, more or less susceptible to climate-related stressors (Nelson 1970; Melzner et al. 2009; Di Santo et al. 2016). Responses to ocean warming and acidification may vary across the life history of organisms, with embryonic and juvenile stages being the most vulnerable (Lear et al. 2019), with acclimation (short vs. long term exposure), and across populations (Donelson et al. 2012; Frommel et al. 2012; Bignami et al. 2013; Harvey et al. 2013; Di Santo 2015; Downie et al. 2020; Gervais et al. 2021). Some long-lived organisms may experience a wide spectrum of environmental change, while others with faster generational turnover may only experience relatively stable conditions within their lifetime (Melzner et al. 2009; Somero 2010). As a consequence, some individuals may be able to acclimate over a relatively long period of time while some populations may rapidly adapt to the changes in their environment across generations favoring physiotypes that are resilient in the new conditions (Munday 2014; Ryu et al. 2018). Organisms can also employ other solutions, such as shifting their geographic range, to respond to environmental challenges (Harvey et al. 2013; Rosa et al. 2014; Browman 2017). Changes in locomotor performance, behavior and geographic range at large and small scale may have cascading and unexpected effects on biotic interactions, community structures and ecosystem functioning (Harvey et al. 2013; Di Santo et al. 2020). Thus, it is important to investigate how performance of different physiotypes may be affected under climate change to better forecast future shifts in marine ecosystems (Di Santo 2016).
Elasmobranchs are ecologically important as they fill many niches across a wide range of biota, from benthic, to coral reefs to open oceans (Wilga and Lauder 2004; Lauder and Di Santo 2015; Flowers et al. 2020). They typically occupy high trophic levels as top or meso-predators, thus playing a key role in the health of marine ecosystems (Rosa et al. 2014; Pistevos et al. 2015). They are characterized by slow growth and relatively long lifespans, and tend to have higher survival rates as juveniles when compared to teleost fishes (Wellington and Victor 1989; Chin et al. 2010). However, their relatively low generational turnover rate makes them particularly vulnerable to rapid changes in the environment (Chin et al. 2010; Rosa et al. 2014). Predictions of responses to climate change often involve modeling of geographic shifts (Parmesan et al. 1999; Saba et al. 2016). However, such projections of poleward movements need to consider swimming capacity and cost of transport. In fact, an efficient locomotor capacity would allow fishes to effectively relocate to environmental refugia (Lauder and Di Santo 2015). It is currently virtually unknown if elasmobranchs’ swimming performance levels may be maintained in response to climate change because only limited studies on relatively few, and especially benthic species have been conducted to date (Wilga and Lauder 2004; Aronson et al. 2007; Papastamatiou et al. 2015; Di Santo 2016; Payne et al. 2018; Lear et al. 2019).
Elasmobranch fishes display a great morphological diversity in body shapes that results in extraordinary locomotor diversity (Rosenberger 2001; Wilga and Lauder 2004; Lauder and Di Santo 2015; Porter et al. 2020). For instance, sharks exhibit an elongated body and swim using mostly their trunk and tail in an undulatory motion, while rays (i.e., skates and rays) have short, stiff head trunk regions forming a disc with slender tail and therefore swim using the pectoral fins (with a few exceptions) in undulatory (i.e., wave-like motion) or oscillatory (i.e., flapping up and down) motions (Rosenberger and Westneat 1999; Rosenberger 2001; Wilga and Lauder 2004). Morphological and kinematic characteristics of the body and fins explain how elasmobranch fishes move and provide cues on important adaptations that are involved with efficient locomotion (Lauder and Di Santo 2015; Di Santo et al. 2021). In this review, we examine the effects of ocean warming and acidification on locomotion in sharks and rays. We also briefly review major studies on the effect of warming and ocean acidification on locomotor performance of teleost fishes because of the paucity of work on elasmobranchs. We have based our review on studies where the effect of ocean warming and/or acidification was assessed on skeletal morphology, locomotor behavior and physiology of elasmobranch fishes. We also discuss features that characterize tolerant physiotypes under the two major climate change stressors in relation to swimming performance.
Locomotor performance
Locomotion is a key process in nearly all fishes, including elasmobranchs, that is involved in vital functions such as reproduction, migration, predator avoidance, ram ventilation, and small-scale movements (Daniel 1984; Sfakiotakis et al. 1999; Lauder and Di Santo 2015). Biomechanics, behavior and energetics of locomotion determine efficiency of movement and maximal performance (for instance, maximum speed during an escape response) under different conditions (Wilga and Lauder 2004; Lauder and Di Santo 2015), and, as such, these are the focus of the review. Two major locomotor behaviors are likely to increase survival rates in elasmobranchs: steady (or sustained) swimming and burst (or escape) performance. During steady swimming, fishes may modulate their speed and can often optimize energetic costs for large scale movements by selecting an optimal velocity (Webb 1994; Saadat et al. 2017; Di Santo et al. 2017b). On the other hand, fishes may undertake energetically costly movements such as fast-starts and bursts to escape a predator, or to capture a prey (Jayne and Lauder 1994; Johnson and Bennett 1995; Fernández et al. 2002; Wen et al. 2018).
Climate change and locomotor performance in teleost fishes
Locomotor performance has undoubtedly contributed significantly to the evolutionary success of fishes (Breder 1926; Lindsey 1978). In fact, fishes display a remarkable diversity of morphology and behaviors to achieve a variety of movements, from long-distance migrations, to fast swimming and escape responses (Lauder 2015). Fish locomotion is a well-studied topic (Bainbridge 1963; Sfakiotakis et al. 1999; Lauder 2015; Saadat et al. 2017; Di Santo et al. 2021), however studies on the single and combined effect of temperature and acidification on many traits that affect locomotor performance are often lacking within the same group of fishes. The scarcity of “full picture” data sets makes the identification of sensitive and tolerant physiotypes complicated. Swimming traits that are often analyzed are maximum speed, metabolic rates, and escape mechanics, but rarely under ocean warming and acidification, and in the same organism (Melzner et al. 2009; Watson et al. 2018).
Temperature changes can affect skeletal morphology, swimming performance and behavior of fishes (von Herbing 2002; Eliason et al. 2011; Hein and Keirsted 2012). Thermal stress during embryonic development is a known teratogenic factor that produces skeletal deformities in teleost fishes (Takle et al. 2005). Warming results in severe malformations in the axial skeleton in species such as the mosquito fish (Gambusia affinis) and the Atlantic salmon (Salmo salar) where the proportion of fish with larger, fused and deformed vertebrae increases with temperature (Takle et al. 2005; Ytteborg et al. 2010; Sassi et al. 2010; Fraser et al. 2015). Likewise, ocean acidification is known to alter the morphology of the skeleton in teleost fishes (Powell et al. 2009; Baumann et al. 2012; Pimentel et al. 2014, 2016; DePasquale et al. 2015). In particular, acidification may lead to fusions, body malformation, and spinal curvatures (i.e., lordosis, scoliosis and kyphosis) (Baumann et al. 2012; Pimentel et al. 2014, 2016). Moreover, an increase in skeletal mineralization has been observed in site-attached fish (Gobius bucchichi) living near CO2 seeps (Mirasole et al. 2020). Malformations of the axial skeleton can alter swimming performance, foraging and predator avoidance in teleosts (Powell et al. 2009). For instance, scoliotic teleost fishes achieve significantly lower swimming speed and are unable to recover quickly from exhaustive swimming when compared to control individuals (Powell et al. 2009).
Temperature also shapes the performance or aerobic scope of swimming (Fry 1947, 1967; Fry and Hart 1948). The concept of aerobic scope was introduced by Fry and Hart (1948) when they quantified the effects of temperature change on maximum and standard metabolic rates in goldfish (Carassius auratus). By subtracting the standard metabolic rate from the maximum, they put forward the idea of the scope for metabolic activity, i.e., the net energy beyond resting that organisms have to be active (Fry and Hart 1948; Farrell 2016). The aerobic scope is a measure of thermal dependence of physiological processes, and it has been used broadly by ecological physiologists to understand changes in performance as a consequence of thermal shifts (Farrell 2016). Performance curves often show that warming increases swimming metabolic rates, but that the peak of maximum oxygen consumption rates typically precedes the peak for resting rates, thus effectively showing a decline in performance at the highest temperatures an individual can tolerate (Fangue et al. 2008; Farrell 2016). Warming can also have a positive effect on swimming performance. In fact, higher temperatures increase muscle efficiency and contractility at high speeds, making swimming much more energetically efficient in teleost larvae (von Herbing 2002). Another study on herring and plaice larvae showed that an increase in muscle contraction performance during escape response leads to faster bursts (Batty and Blaxter 1992). On the other hand, the same study noted that other traits, such as tail amplitude and stride length, are not significantly affected by temperature (Batty and Blaxter 1992). Several studies have shown an increase in fast-start and burst swimming in adult fishes that resulted in enhanced escape performance with warming (Johnston et al. 1991; Wilson et al. 2001; Fernández et al. 2002; Lyon et al. 2008). The effect of temperature on oxygen consumption during steady swimming has been investigated using many species, from salmonids to Antarctic fishes (some examples: Brett 1967; Wilson et al. 2001; Jain and Farrell 2003; Fangue et al. 2008). In most studies warming increases swimming endurance and oxygen consumption up to the organism’s thermal limit where performance rapidly declines (Brett 1964, 1967; Johnston et al. 1991; Steinhausen et al. 2008; Eliason et al. 2011; McDonnell and Chapman 2016). In some species temperature does not have a significant effect on swimming energetics or speed, unless the effect of low temperature was tested (Johnston et al. 1991; Fangue et al. 2008).
Ocean acidification has been shown to reduce swimming performance in some fish species but responses to ocean acidification are often complex (Munday 2014; Browman 2017; Clark et al. 2020). In fact, studies have reported an increase, a decrease, or no significant change in aerobic scope associated with swimming at high pCO2 (Munday et al. 2009; Couturier et al. 2013; Rummer et al. 2013; Esbaugh et al. 2016). High pCO2 levels decrease maximum speed during steady and burst swimming, and distance traveled (Allan et al. 2014; Watson et al. 2018). During escape responses, fishes bend their body into a “c”, i.e., the first stage, and then produce a fast burst to move away from the stimulus, i.e., the second stage (Jayne and Lauder 1994; Witt et al. 2015). Even though the bending angle during the first stage of the escape response may not be affected by acidification, fishes may be less responsive to stimuli that trigger the escape response (Wang et al. 2017).
To predict the long-term effects of ocean warming and acidification on swimming performance of fishes, it is important to consider whether physiological and behavioral traits associated with locomotion may adjust, or acclimate, within the time frame in which these changes are occurring in the environment, i.e., a few generations (Donelson et al. 2012). Although there is a dearth of studies that have evaluated the effects of warming and acidification across fish generations, metabolic rates and swimming kinematics are already known to be compensated during environmental shifts in some species (Schade et al. 2014; Shama et al. 2014; Munday 2014; Veilleux et al. 2015). Such transgenerational acclimation could be achieved via changes in the epigenetic state of parental generation that is transferred to next generation by producing offspring that maintain a similar performance in the new environment, or a maternal effect that provides, for example, different amount of nutrition to the offspring through the yolk (Shama et al. 2014; Veilleux et al. 2015; Ryu et al. 2018). However, there may be limitations to the traits that respond to transgenerational acclimation. Some biomechanical traits such as turning angle during escape response are affected by increase in pCO2 but do not seem to acclimate even when the parents are exposed to the same level of acidification (Munday 2014). On the other hand, exposure to acidification across generations might produce a carry-over effect thus further reducing performance and survival (Munday 2014). These early studies suggest that transgenerational acclimation may be inconsequential, reduce or exacerbate the effect of ocean acidification and warming on different morphological, physiological, and behavioral traits associated with swimming. Understanding the outcome of the single and combined effects of ocean warming and acidification on performance traits is a priority for studies that aim to realistically predict complex responses of organisms to changes in the environment. Even though just a few studies looked at transgenerational acclimation in teleost fishes, to our knowledge no study to date has quantified it on any elasmobranch species.
Effect of ocean warming on locomotor performance in elasmobranchs
Temperature is known to have a profound effect on nearly every physiological process, including metabolic rates (for example, Hawkins and Day 1996; Rosa et al. 2014; Gervais et al. 2018), but there is a paucity of data on the effect of temperature on morphology of elasmobranchs, beyond body size, and swimming performance (Di Santo 2015, 2019). In one study, Di Santo (2019) investigated the effect of ocean warming on skeletal mineralization in a batoid fish. Little skate (Leucoraja erinacea) embryos were exposed to temperature projected for the year 2100 (Di Santo 2019). Warming had a negative effect on the mineralization of the pectoral fins as the density of apatite decreased (Di Santo 2019). One of the possible causes of a reduction in mineralization in the pectoral fins of skates may be attributed to a mismatch between high growth in juveniles at higher temperatures and mineralization processes that might proceed at lower rates (Di Santo 2019). High stiffness of the cartilage provides an efficient energy transfer during locomotion (Dean and Summers 2006; Porter and Long 2010). A decrease in mineralization of the cartilage in the pectoral fins produces higher flexibility during movement but the fish needs to actively stiffen the fins to achieve higher swimming speeds, thus increasing metabolic costs during swimming (Di Santo et al. 2017a).
Warming also correlates with a smaller body size, a phenomenon so widespread across terrestrial and aquatic organisms that it is known as one of the universal responses to climate change (Gardner et al. 2011; Forster et al. 2012; Baudron et al. 2014). A smaller body size has been observed in juvenile epaulette shark, Hemiscyllium ocellatum, (Gervais et al. 2018) while juvenile little skates showed a lower body condition when exposed to higher temperatures (Di Santo 2015). In little skates, local adaptation seems to have a strong effect on the temperature-size relationship. Skates from the areas where temperature fluctuates frequently seem to be less affected by warming than conspecific living in more stable environments (Di Santo 2015, 2016). Populations of Port Jackson sharks (Heterodontus portusjacksoni) also exhibit differences in metabolic rates in response to temperature, even though swimming performance was not different (Gervais et al. 2021). Smaller elasmobranch and teleosts fishes tend to be more tolerant to high temperatures and are assumed to perform better at higher temperatures that their larger conspecifics (Di Santo 2016; McKenzie et al. 2020). In particular, smaller skates escape more intensely (burst/min) and can recover faster after a chasing experiment when compared to larger conspecifics at the same temperatures (Di Santo 2016). Even though smaller fishes are known to perform better under increasing temperatures, a small body size can significantly impair long-distance locomotor capacity by increasing cost of transport in skates (Dulvy et al. 2014; Lauder and Di Santo 2015; Di Santo and Kenaley 2016; Di Santo et al. 2017b) and sharks (Carlson et al. 2004).
Warming is associated with a reduced aerobic scope for activity and increased stress (Schwieterman et al. 2019). It has been suggested that some fish species that live close to their thermal optimum may experience a reduction in the amount of available energy for aerobic activities, such as locomotion, with warming (Rummer et al. 2014). Elasmobranch species that are fast swimmers and obligate ram ventilators have the highest measures of routine metabolic rates to maintain, for example, higher digestion rates and, in some cases, heterothermy, and therefore are expected to have limited amount of energy to cope with environmental challenges than less active species (Carlson et al. 2004). On the other hand, even though locomotor efficiency per se may be reduced, these elasmobranchs may be more likely to relocate to a more suitable area by swimming faster (Wilga and Lauder 2004; Chin et al. 2010; Lauder and Di Santo 2015). However, it is difficult to establish the effect of temperature on aerobic scope as many studies have now shown that the maximum metabolic rates in fishes are affected by digestion, swimming capacity, stress, and other masked abiotic factors such as oxygen levels (Roche et al. 2013; Di Santo and Lobel 2016; Rummer et al. 2016; Esbaugh et al. 2021; Pauly 2021). In a previous study, Di Santo (2015) argued that an increase in active metabolic rate as a consequence of warming, should not be always considered an advantage. In fact, determining the actual maximum metabolic rate is difficult and it relies heavily on the technique used to test swimming performance (Roche et al. 2013; Rummer et al. 2016), so it is possible that researchers have been routinely measuring the elevated costs of activity with climate stressors rather than an increase in aerobic scope. Conclusions on the effect of temperature and other stressors on aerobic scope should therefore be cautious.
Predictions of future responses to climate change often include shifts in geographic ranges, while small scale alterations in habitat use have received less attention. Elasmobranchs can utilize the diel abiotic and geographical variability in their environment to decrease costs of locomotion (Papastamatiou et al. 2021). Sharks and rays are known to exploit the thermal heterogeneity in their environment by selecting different temperatures throughout the day, a behavior known as thermotaxis (Fangue and Bennett 2003; Wallman and Bennett 2006; DiGirolamo et al. 2012; Speed et al. 2012). Elasmobranchs may select specific temperatures to enhance physiological processes, from swimming performance to digestion to reproduction (Wallman and Bennett 2006; Di Santo and Bennett 2011a; Papastamatiou et al. 2015), while others do not seem to adjust to changes in the environment by thermoregulation (Nay et al. 2021). One example of thermoregulation is the trade-off between optimal foraging and thermal habitats (Sims et al. 2006; Sims 2010), where sharks and rays hunt in warmer waters, to maximize muscle performance, and rest in cool waters, to slow down the passage of food across the absorptive surfaces and increase nutrient uptake, thus reducing the daily energy costs (Sims et al. 2006; Sims 2010) and increasing digestive efficiency (Di Santo and Bennett 2011a, b). It is possible therefore that elasmobranchs may not shift their latitudinal range with warming, but rather select deeper cooler waters to enhance physiological processes. These small scales movements may be just as significant to the survival of sharks and rays to ocean warming than poleward relocations. Seasonal shifts towards deeper water are already occurring for batoids at high latitudes (Stebbing et al. 2002; Perry et al. 2005; Dulvy et al. 2008b; Lauder and Di Santo 2015). Perry et al. (2005) quantified a higher mean depth in the distribution of the ray Leucoraja naevus that correlates with warming trends. In this multi-species study, fishes that shifted their geographic range tended to have faster life history compared to non-shifting species (Perry et al. 2005). Dulvy et al. (2008b) suggested that there is evidence for coherent deepening of fish in response to climate change and found that the shift in depth was more pronounced than the latitudinal shift in benthic elasmobranchs. Although a shift in distribution is possible, bathymetry (deep waters), the expansion of oxygen minimum zones (Di Santo et al. 2016; Vedor et al. 2021), potential decoupling of current predator–prey dynamics and trophic structure (Deutsch et al. 2015), and behavioral characteristics of movement (philopatry, restricted home ranges) may hinder the capacity to relocate to more favorable refugia (Aronson et al. 2007; Dulvy et al. 2014).
Effect of ocean acidification on locomotor performance in elasmobranchs
Elasmobranch fishes evolved during the Devonian Period when atmospheric CO2 was greater than today’s levels (Johnson et al. 2016; Rosa et al. 2017a). This has led to the hypothesis that elasmobranch fishes may be able to tolerate ocean acidification, and it was not until recently that experimental data showed that high levels of pCO2 can affect elasmobranch fishes directly by causing morphological, behavioral and physiological anomalies (Pistevos et al. 2015; Rosa et al. 2017a). Di Santo (2019) evaluated the effect of ocean acidification on mineralization of the skeleton of little skates that were developmentally acclimated to pH levels expected by year 2100. Levels of pCO2 ~ 1100 µatm increased mineralization of the crura (modified pelvic fins that some rays use to walk underwater) and jaws in little skates (Di Santo 2019). Skates might even gain an advantage as high mineralization of the crura is a good predictor of the ability of batoids to walk underwater (Koester and Spirito 2003; Macesic and Summers 2012; Bilecenoglu and Ekstrom 2013). Apatite in the crura increased with pCO2 with no significant effect of temperature, implying that future ocean acidification may favor walking in benthic batoids regardless of warming (Di Santo 2019). On the other hand, a denser skeleton increases the weight of the skeleton thus reducing buoyancy which, in turn, increases energetic costs of swimming (Di Santo 2019). Elasmobranch do not have a swim bladder and need to swim in order to create hydrodynamic lift and even hover in the water column. Therefore, a heavier skeleton is expected to increase costs of locomotion at any speed (Di Santo et al. 2017b). While external features such as teeth and denticles are not affected by pCO2 levels expected by the end of the century (Green and Jutfelt 2014; Di Santo 2019), higher levels projected for the year 2300 are known to cause corrosion of denticles in sharks (Dziergwa et al. 2019). Denticle size and morphology are important during locomotion as these features may increase efficiency by reducing the friction between the skin and the water (Oeffner and Lauder 2012; Wen et al. 2014; Domel et al. 2018). Studies have not reported any body malformation linked to acidification in elasmobranchs yet, and it has been suggested that elasmobranch embryos might be protected from acidification during the earliest stages of development while inside their mothers or in a closed eggcase (Leonard et al. 1999; Di Santo 2015). Additionally, elasmobranchs have direct development, while many teleosts go through a larval phase that is considered vulnerable to acidification (Frommel et al. 2012; Bignami et al. 2013).
The combined effect of ocean warming and acidification on locomotor performance in elasmobranchs
Ocean warming and ocean acidification will occur simultaneously in oceans, and organisms, including elasmobranchs, may be negatively impacted by the interactive effects of these stressors (Byrne and Przeslawski 2013; Boyd et al. 2015; Di Santo 2015; Pistevos et al. 2017). Di Santo (2015, 2016, 2019) provides the only series of studies that have looked at single and combined effects of ocean warming and acidification on different performance traits for the same elasmobranch species across ontogeny. First, ocean warming and acidification interacted during mineralization of the skeleton of skates. Acidification increased density of the crura and the jaws at control temperature; however, when combined with warming, acidification had no significant effect on the mineralization of the jaws (Di Santo 2019). On the other hand, warming did not reduce the effect of acidification on the mineralization of the crura (Di Santo 2019). Complex and non-directional results are becoming common in studies of ocean acidification and warming across organisms (Kroeker et al. 2010, 2013; Schlegel et al. 2012).
Denser skeletal parts as a consequence of acidification and less mineralized pectoral fins as a consequence of warming can have a profound effect on locomotor performance. In fact, both a heavier skeleton that reduces buoyancy and less stiff pectoral fins that need to be actively stiffened using muscles, increase the costs of locomotion in skates (Di Santo 2016; Di Santo et al. 2017a, b). During a chasing experiment, combined warming and acidification prolonged the time to recover from intense exercise (Di Santo 2016). A longer recovery time and higher energetic costs to escape may make skates vulnerable to predators as they need to pay off the oxygen debt accumulated during intense sequential bursts, and they may need to explore the environment to seek additional food (Di Santo 2015, 2016). Moreover, a few studies have shown that hunting efficacy in sharks may be impaired by the effect of ocean acidification and warming, while other studies found no effect (Pistevos et al. 2015, 2017; Heinrich et al. 2016; Rosa et al. 2017b; Gervais et al. 2018). Elevated temperature increased swimming activity while searching for food and reduced the time used by elasmobranchs to digest (Di Santo and Bennett 2011a; Pistevos et al. 2017), but acidification can reduce elasmobranchs’ ability to use olfactory cues and increased the time to find food even in combination with elevated temperature (Pistevos et al. 2017). Port Jackson sharks under warming scenarios increased the time spent near chemical cues for prey but pCO2 reduced the attraction to food (Pistevos et al. 2017). Pistevos et al. (2015) reported that it took four times longer for sharks to detect prey under elevated pCO2. When combined with elevated temperature, sharks reduced the time to locate the prey by a third but was still significantly longer than the time needed by control individuals (Pistevos et al. 2015). High pCO2 may reduce elasmobranchs’ ability to detect prey perhaps because of a dysfunction of the GABAA receptor (Hamilton et al. 2014), thus making some elasmobranch species slower and less successful hunters. These studies suggest that ocean warming and acidification may have the potential to reduce hunting abilities in some elasmobranchs by disrupting olfactory capacity, thus requiring them to rely more on vision and electroreception to find prey (Pistevos et al. 2015).
The effect of ocean warming and acidification can be modulated by the local environment a population or individual has experienced. For instance, when comparing the escape performance of little skate from two adjacent locations, the Gulf of Maine and the Georges Bank, under simulated warming and acidification conditions, the smaller skates from the Georges Bank were able to escape for a longer period of time and recovered faster from exercise (Di Santo 2016). It is unclear however, whether there was a maternal effect given by the periodic exposure of egg-laying females to low-pH upwelling waters in the Georges Bank (Mavor and Bisagni 2001; Raven et al. 2005) or a smaller body size favored locomotor performance in that particular population of skates (Di Santo 2016). Additional studies are needed to understand the role of local adaptation and size on locomotor performance in elasmobranchs under climate change scenarios.
Consequences of alterations in locomotion efficiency on geographic shifts
Low locomotor efficiency is associated with a reduced capacity for migrating, hunting, and effectively utilizing the environment through small-scale movements (Lauder 2015; Lauder and Di Santo 2015). At a global scale, fishes migrate to relocate to more favorable conditions and find thermal refugia (Stebbing et al. 2002; Perry et al. 2005; Dulvy et al. 2008b). Even though there are no apparent geographic barriers to marine dispersal (Aronson et al. 2007), a reduction in locomotion efficiency can limit elasmobranch movement and the capacity to relocate.
Large scale migrations to find thermal refugia occur as a poleward expansion or towards deeper waters (Stebbing et al. 2002; Perry et al. 2005; Dulvy et al. 2008b; Lauder and Di Santo 2015). Range expansions and poleward shifts assume that (1) species are able to migrate and (2) species are able to find new suitable habitats. Some elasmobranchs show strong philopatry or are geographically restricted, making it hard for them to colonize new habitat (Dulvy et al. 2008b; Lauder and Di Santo 2015; Kneebone et al. 2020). In particular, skate populations show high philopatry, and even when a nearby population is locally extirpated, they are unlikely to expand their range and repopulate that area (Dulvy et al. 2014). In some cases long distance relocations might be hindered in deep waters as benthic elasmobranchs cannot stop and rest on the bottom of the oceans to recover (Dulvy et al. 2008a, b). In fact, high hydrostatic pressure increases the concentration of trimethylamine-N-oxide (TMAO) in the body fluids of elasmobranchs (Priede et al. 2006; Laxson et al. 2011; Yancey et al. 2014, 2018). TMAO is a naturally occurring osmolyte that stabilizes the three-dimensional structure of proteins (Yancey et al. 1982). In deep waters high pressure causes muscle TMAO to increase and urea to decrease. TMAO amplifies the inward osmotic gradient which becomes fatal as the kidneys are unable to compensate (Aronson et al. 2007). On the other hand, TMAO production plateaus at depths of around 3000 m, hence elasmobranchs may be unable to accumulate sufficient TMAO to counteract pressure (Laxson et al. 2011).
Future warming is forecasted to be most pronounced in the Southern ocean (Intergovernmental Panel on Climate Change 2015) and elevated temperatures are expected to facilitate the introduction and establishment of new species (Aronson et al. 2007). Thus, a significant latitudinal shifting of elasmobranchs is expected to be towards shallow waters in Antarctica in the southern hemisphere (Aronson et al. 2007). The continental shelf around Antarctica is deeper than 3000 m and when migrating to Antarctica, elasmobranchs must swim continuously to cross the deep ocean (Aronson et al. 2007). Fast swimming pelagic sharks have the ability to swim continuously and have a high probability of crossing the depths to reach Antarctica, while benthic sharks, skates and most rays lack the capacity to swim continuously thus facing physiological challenges while crossing the continental shelf (Aronson et al. 2007). Additionally, benthic elasmobranchs tend to have small body size (Carrier et al. 2004) which corresponds to high cost of transport regardless of swimming metabolic rates, thus limiting large scale migrations (Lauder and Di Santo 2015). With ocean acidification increasing skeletal mineralization, we might expect that benthic elasmobranchs will develop an even more sluggish behavior, making their migrations paths less likely to occur. Therefore, we suggest that morphological and physiological traits that contribute to swimming ability may be considered important factors when modelling future large-scale shifts in elasmobranch fishes.
Identifying tolerant elasmobranch physiotypes under climate change
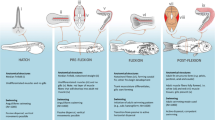
Elasmobranch fishes have survived all five major mass extinction events that have occurred over their evolution (last 400 million years), making them one of the most resilient marine groups (Rosa et al. 2017a). However, because they have relatively long generation time and many of them are currently overfished, they are considered one of the most threatened marine groups (Field et al. 2009; Chin et al. 2010). Current rates of change in temperature and pH are much faster than those they experienced during previous climatic shifts (Hoffmann and Sgrò 2011). Specifically, the rate of warming and acidification combined with other stressors (e.g., habitat destruction, prey availability, exploitation) may render their management and protection difficult (Baum et al. 2003; Dulvy et al. 2008a, 2014; Dawson et al. 2011; Birkmanis et al. 2020). As it is unlikely that elasmobranchs may be able to adapt to rapid changes in the environment through evolutionary processes, many species may adjust to these synergistic changes through alterations in behavior (such as shifts in habitat utilization) or distribution. To be able to forecast future outcomes and strategize on different measures to implement to manage wild populations, it is important to identify key traits that impact swimming performance under climate change scenarios (Fig. 1).
Physiotypes that are able to swim fast have an advantage when shifting their geographic distribution and exploiting new thermal gradients and areas. These are for example heterothermic (lamnid) sharks that can usually swim faster and for longer periods than poikilothermic species (Carrier et al. 2004; Sims 2010). Morphological features such as tapered fins and streamlined bodies also favor swimming efficiency, and are characteristic of elasmobranch physiotypes that already show wide geographic distribution (Lauder and Di Santo 2015). Warmer habitat physiotypes from lower latitudes may be more likely to fare well in future climate change, as they may experience a smaller increase in temperature compared to species at higher latitudes (Perry et al. 2005; Gervais et al. 2018), however hypoxic events in these areas could reduce performance (Parsons and Carlson 1998; Carlson and Parsons 2001; Di Santo et al. 2016; Crear et al. 2019; Esbaugh et al. 2021). Species experiencing fluctuating environments across their geographic range might be better able to adjust to climate change (Hoffmann and Sgrò 2011; Seibel et al. 2012; Di Santo 2016; Johnson and Hofmann 2020). Such species can either exhibit locally adapted populations that respond differently to stressors (species with reduced dispersal capacity) or global distribution (high swimming and dispersal capacity) (Dulvy et al. 2008b; Field et al. 2009; Booth et al. 2011; Lauder and Di Santo 2015). Physiotypes with widespread distribution usually have a higher tolerance to novel environments because they have already experienced a variety of climate and habitat conditions within their home range (Field et al. 2009). Furthermore, a wide geographic range usually correlates positively with body size (Dulvy et al. 2014). Bigger elasmobranchs exhibit lower costs of transport compared to smaller ones (Lauder and Di Santo 2015), and have the potential to effectively respond to environmental stressors through changes in distribution. Yet, physiotypes characterized by smaller body size could have an advantage, even if not during migrations. In fact, smaller fishes tend to be more tolerant to changes in temperature compared to bigger ones (Di Santo and Lobel 2017; McKenzie et al. 2020), and are less sensitive to warming and ocean acidification (Wittmann and Pörtner 2013; Di Santo and Lobel 2016; Di Santo 2016). Smaller and benthic elasmobranchs typically show low metabolic rates during swimming, and are known to exploit the variation in their habitats by shuttling across thermal gradients (Wallman and Bennett 2006; Di Santo and Bennett 2011a; Papastamatiou et al. 2015; Flowers et al. 2020). Small scale movements may be important for the survival of many benthic species that could not otherwise relocate to more suitable areas. For instance, elasmobranchs that live in thermally variable environments may continue to select specific temperatures to enhance different physiological processes, for example, higher temperatures to enhance high energy bursts to hunt, and low temperatures to save energy during digestion or recovery from exercise (Hight and Lowe 2007; Meese and Lowe 2019; Silva-Garay and Lowe 2021). This behavior is already observed in several benthic sharks and rays and may become even more widespread with ocean warming. Ocean acidification is likely to increase the energy costs during swimming, and to prolong recovery times after bursts, but is also likely to increase body size and favor underwater walking by increasing mineralization of pelvic fins which is observed in several benthic rays and sharks already (Bilecenoglu and Ekstrom 2013; Jung et al. 2018; Di Santo 2019; Wheeler et al. 2020, 2021).
Conclusions and future directions
Locomotor efficiency is a major trait determining the capacity of elasmobranchs to fare well in climate change, however very few studies have examined how it will be affected by ocean warming, acidification and other related stressors like hypoxia. Ocean warming and acidification exert both single, synergistic and antagonistic effects on elasmobranch locomotion efficiency by affecting skeletal mineralization, increasing metabolic rates during swimming, and prolonging the time it takes to recover from exercise, and to locate food (Rosa et al. 2014, 2017b; Pistevos et al. 2015; Di Santo 2016; Gervais et al. 2021). These morphological and physiological impairments, in combination with hypoxia, pollution, fishing and habitat destruction, may act to reduce elasmobranch survival (Parsons and Carlson 1998; Carlson and Parsons 2001; Field et al. 2009; Dulvy et al. 2014; Crear et al. 2019; Bouyoucos et al. 2019). However, there is dearth of empirical studies on the capacity of elasmobranchs to swim efficiently under simulated future ocean conditions. We suggest that future studies quantify the single and combined effect of ocean acidification, warming and other stressors such as hypoxia on multiple locomotor behaviors within the same species of sharks and rays to identify potential trade-offs (Fig. 2). In particular, more studies are needed on the effect of these stressors on burst and steady swimming energetics and biomechanics (including metabolic rates, maximum and optimal swimming speeds, endurance, tail beat frequency, head and tail amplitude, waves speed and length, body angle and curvature) and ontogenetic studies to understand long term consequences on morphology (body size, skeletal mineralization, fin flexibility and shape) and swimming mechanics. Moreover, it is just as important to quantify intraspecific variation in responses to stressors (Feder et al. 1987; Pough 1988). Intraspecific variation in performance can clarify which traits make individuals more or less efficient during swimming under different environmental conditions. Finally, more studies should be conducted to understand the effect of climate change on swimming in larger elasmobranchs. These species are typically more difficult to study in laboratory settings but technological advances in field equipment paired with laboratory testing (Whitney et al. 2007; Lawson et al. 2019; Papastamatiou et al. 2021) can allow us to understand how sharks and rays may exploit the variation in their environment to save energy during locomotion.
Major knowledge gaps on the effect of climate changes stressors on elasmobranch locomotion. Here, we outline the major climate-related stressors that may affect locomotor performance in elasmobranchs and the intrinsic characteristics of physiotypes that can modulate individual responses to changes in the environment, such as local adaptation, acclimation capacity, intersex variation in responses, parental and transgenerational effects
References
Allan BJ, Miller GM, McCormick MI et al (2014) Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc R Soc B Biol Sci 281:20132179
Arneth A, Shin Y-J, Leadley P et al (2020) Post-2020 biodiversity targets need to embrace climate change. Proc Natl Acad Sci 117:30882–30891
Aronson RB, Thatje S, Clarke A et al (2007) Climate change and invasibility of the antarctic benthos. Annu Rev Ecol Evol Syst 38:129–154. https://doi.org/10.1146/annurev.ecolsys.38.091206.095525
Bainbridge R (1963) Caudal fin and body movement in the propulsion of some fish. J Exp Biol 40:23–56
Batty RS, Blaxter JHS (1992) The effect of temperature on the burst swimming performance of fish larvae. J Exp Biol 170:187–201. https://doi.org/10.1242/jeb.170.1.187
Baudron AR, Needle CL, Rijnsdorp AD, Tara Marshall C (2014) Warming temperatures and smaller body sizes: synchronous changes in growth of North sea fishes. Glob Change Biol 20:1023–1031
Baum JK, Myers RA, Kehler DG et al (2003) Collapse and conservation of shark populations in the Northwest Atlantic. Science 299:389–392
Baumann H, Talmage SC, Gobler CJ (2012) Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat Clim Change 2:38
Bignami S, Sponaugle S, Cowen RK (2013) Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob Change Biol 19:996–1006
Bilecenoglu M, Ekstrom L (2013) Pelvic fin walking and punting behaviour of Raja radula Delaroche, 1809 observed in the Sea of Marmara. Mediterr Mar Sci 14:158–161
Birkmanis CA, Partridge JC, Simmons LW et al (2020) Shark conservation hindered by lack of habitat protection. Glob Ecol Conserv 21:e00862
Booth DJ, Bond N, Macreadie P (2011) Detecting range shifts among Australian fishes in response to climate change. Mar Freshw Res 62:1027–1042
Bouyoucos IA, Simpfendorfer CA, Rummer JL (2019) Estimating oxygen uptake rates to understand stress in sharks and rays. Rev Fish Biol Fish 29:297–311. https://doi.org/10.1007/s11160-019-09553-3
Bouyoucos IA, Watson S-A, Planes S et al (2020) The power struggle: assessing interacting global change stressors via experimental studies on sharks. Sci Rep 10:19887. https://doi.org/10.1038/s41598-020-76966-7
Boyd PW, Lennartz ST, Glover DM, Doney SC (2015) Biological ramifications of climate-change-mediated oceanic multi-stressors. Nat Clim Change 5:71–79
Breder CM (1926) The locomotion of fishes. Zoologica 4:159–291
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21:1183–1226. https://doi.org/10.1139/f64-103
Brett JR (1967) Swimming performance of sockeye salmon (Oncorhynchus nerka) in relation to fatigue time and temperature. J Fish Res Board Can 24:1731–1741. https://doi.org/10.1139/f67-142
Browman HI (2017) Towards a broader perspective on ocean acidification research. ICES J Mar Sci 74:889–894
Byrne M, Przeslawski R (2013) Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr Comp Biol 53:582–596
Carlson JK, Parsons GR (2001) The effects of hypoxia on three sympatric shark species: physiological and behavioral responses. Environ Biol Fishes 61:427–433
Carlson J, Goldman K, Lowe C (2004) Metabolism, energetic demand, and endothermy. In: Musick John, Carrier Jeffrey, Heithaus Michael (eds) Biology of sharks and their relatives. CRC Press, pp 203–224. https://doi.org/10.1201/9780203491317.ch7
Carrier JC, Musick JA, Heithaus MR (eds) (2004) Biology of sharks and their relatives. CRC Press, Boca Raton
Chin A, Kyne PM, Walker TI, McAuley RB (2010) An integrated risk assessment for climate change: analysing the vulnerability of sharks and rays on Australia’s Great Barrier Reef. Glob Change Biol 16:1936–1953
Clark TD, Raby GD, Roche DG et al (2020) Ocean acidification does not impair the behaviour of coral reef fishes. Nature 577:370–375. https://doi.org/10.1038/s41586-019-1903-y
Couturier CS, Stecyk JA, Rummer JL et al (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166:482–489
Crear DP, Brill RW, Bushnell PG, Latour RJ, Schwieterman GD, Steffen RM, Weng KC (2019) The impacts of warming and hypoxia on the performance of an obligate ram ventilator. Conserv Physiol. https://doi.org/10.1093/conphys/coz026
Daniel TL (1984) Unsteady aspects of aquatic locomotion. Am Zool 24:121–134
Dawson TP, Jackson ST, House JI et al (2011) Beyond predictions: biodiversity conservation in a changing climate. Science 332:53–58
Dean MN, Summers AP (2006) Mineralized cartilage in the skeleton of chondrichthyan fishes. Zoology 109:164–168
DePasquale E, Baumann H, Gobler CJ (2015) Vulnerability of early life stage Northwest Atlantic forage fish to ocean acidification and low oxygen. Mar Ecol Prog Ser 523:145–156
Deutsch C, Ferrel A, Seibel B et al (2015) Climate change tightens a metabolic constraint on marine habitats. Science. https://doi.org/10.1126/science.aaa1605
Di Santo V (2015) Ocean acidification exacerbates the impacts of global warming on embryonic little skate, Leucoraja erinacea (Mitchill). J Exp Mar Biol Ecol 463:72–78. https://doi.org/10.1016/j.jembe.2014.11.006
Di Santo V (2016) Intraspecific variation in physiological performance of a benthic elasmobranch challenged by ocean acidification and warming. J Exp Biol 219:1725–1733. https://doi.org/10.1242/jeb.139204
Di Santo V (2019) Ocean acidification and warming affect skeletal mineralization in a marine fish. Proc R Soc B 286:20182187. https://doi.org/10.1098/rspb.2018.2187
Di Santo V, Bennett WA (2011a) Is post-feeding thermotaxis advantageous in elasmobranch fishes? J Fish Biol 78:195–207. https://doi.org/10.1111/j.1095-8649.2010.02853.x
Di Santo V, Bennett WA (2011b) Effect of rapid temperature change on resting routine metabolic rates of two benthic elasmobranchs. Fish Physiol Biochem 37:929–934. https://doi.org/10.1007/s10695-011-9490-3
Di Santo V, Kenaley CP (2016) Skating by: low energetic costs of swimming in a batoid fish. J Exp Biol 219:1804–1807. https://doi.org/10.1242/jeb.136358
Di Santo V, Lobel PS (2016) Size affects digestive responses to increasing temperature in fishes: physiological implications of being small under climate change. Mar Ecol 37:813–820. https://doi.org/10.1111/maec.12358
Di Santo V, Lobel PS (2017) Body size and thermal tolerance in tropical gobies. J Exp Mar Biol Ecol 487:11–17. https://doi.org/10.1016/j.jembe.2016.11.007
Di Santo V, Tran AH, Svendsen JC (2016) Progressive hypoxia decouples activity and aerobic performance of skate embryos. Conserv Physiol 4(1):cov067. https://doi.org/10.1093/conphys/cov067
Di Santo V, Blevins EL, Lauder GV (2017a) Batoid locomotion: effects of speed on pectoral fin deformation in the little skate, Leucoraja erinacea. J Exp Biol 220:705–712. https://doi.org/10.1242/jeb.148767
Di Santo V, Kenaley CP, Lauder GV (2017b) High postural costs and anaerobic metabolism during swimming support the hypothesis of a U-shaped metabolism: speed curve in fishes. Proc Natl Acad Sci 114:13048–13053. https://doi.org/10.1073/pnas.1715141114
Di Santo V, O’Boyle LA, Saylor RK et al (2020) Coral loss alters guarding and farming behavior of a Caribbean damselfish. Mar Biol 167:120. https://doi.org/10.1007/s00227-020-03726-6
Di Santo V, Goerig E, Wainwright DK et al (2021) Convergence of undulatory swimming kinematics across a diversity of fishes. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2113206118
DiGirolamo A, Gruber S, Pomory C, Bennett W (2012) Diel temperature patterns of juvenile lemon sharks Negaprion brevirostris, in a shallow-water nursery. J Fish Biol 80:1436–1448
Domel AG, Domel G, Weaver JC et al (2018) Hydrodynamic properties of biomimetic shark skin: effect of denticle size and swimming speed. Bioinspiration Biomim 13:056014
Donelson JM, Munday PL, McCormick MI, Pitcher CR (2012) Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat Clim Change 2:30–32. https://doi.org/10.1038/nclimate1323
Downie AT, Illing B, Faria AM, Rummer JL (2020) Swimming performance of marine fish larvae: review of a universal trait under ecological and environmental pressure. Rev Fish Biol Fish 30:93–108. https://doi.org/10.1007/s11160-019-09592-w
Dulvy NK, Baum JK, Clarke S et al (2008a) You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat Conserv Mar Freshwat Ecosyst 18:459–482
Dulvy NK, Rogers SI, Jennings S et al (2008b) Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J Appl Ecol 45:1029–1039. https://doi.org/10.1111/j.1365-2664.2008.01488.x
Dulvy NK, Fowler SL, Musick JA et al (2014) Extinction risk and conservation of the world’s sharks and rays. eLife 3:e00590. https://doi.org/10.7554/eLife.00590
Dupont S, Pörtner H (2013) Marine science: get ready for ocean acidification. Nature 498:429
Dziergwa J, Singh S, Bridges CR et al (2019) Acid-base adjustments and first evidence of denticle corrosion caused by ocean acidification conditions in a demersal shark species. Sci Rep 9:1–10
Eliason EJ, Clark TD, Hague MJ et al (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332:109–112
Esbaugh AJ, Ern R, Nordi WM, Johnson AS (2016) Respiratory plasticity is insufficient to alleviate blood acid–base disturbances after acclimation to ocean acidification in the estuarine red drum, Sciaenops ocellatus. J Comp Physiol B 186:97–109
Esbaugh AJ, Ackerly KL, Dichiera AM, Jr Negrete B (2021) Is hypoxia vulnerability in fishes a by-product of maximum metabolic rate? J Exp Biol 224:jeb232520
Fangue NA, Bennett WA (2003) Thermal tolerance responses of laboratory-acclimated and seasonally acclimatized Atlantic stingray, Dasyatis sabina. Copeia 2003:315–325. https://doi.org/10.1643/0045-8511(2003)003[0315:TTROLA]2.0.CO;2
Fangue NA, Mandic M, Richards JG, Schulte PM (2008) Swimming performance and energetics as a function of temperature in killifish Fundulus heteroclitus. Physiol Biochem Zool 81:389–401. https://doi.org/10.1086/589109
Farrell AP (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88:322–343. https://doi.org/10.1111/jfb.12789
Feder ME, Feder ME, Bennett AF et al (1987) New directions in ecological physiology. Cambridge University Press, New York, p 364
Fernández DA, Calvo J, Wakeling J et al (2002) Escape performance in the sub-Antarctic notothenioid fish Eleginops maclovinus. Polar Biol 25:914–920. https://doi.org/10.1007/s00300-002-0435-2
Field IC, Meekan MG, Buckworth RC, Bradshaw CJA (2009) Chapter 4 susceptibility of sharks, rays and chimaeras to global extinction. Elsevier. https://doi.org/10.1016/S0065-2881(09)56004-X
Flowers KI, Heithaus MR, Papastamatiou YP (2020) Buried in the sand: uncovering the ecological roles and importance of rays. Fish Fish. https://doi.org/10.1111/faf.12508
Forster J, Hirst AG, Atkinson D (2012) Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc Natl Acad Sci 109:19310–19314
Fraser TWK, Hansen T, Fleming MS, Fjelldal PG (2015) The prevalence of vertebral deformities is increased with higher egg incubation temperatures and triploidy in Atlantic salmon Salmo salar L. J Fish Dis 38:75–89. https://doi.org/10.1111/jfd.12206
Frommel AY, Maneja R, Lowe D et al (2012) Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Change 2:42–46. https://doi.org/10.1038/nclimate1324
Fry FEJ (1947) Effects of the environment on animal activity. Publ ont Fish Res Lab 55:1–62
Fry FJ (1967) Responses of vertebrate poikilotherms to temperature. Thermobiology Academic Press, New York
Fry FEJ, Hart JS (1948) The relation of temperature to oxygen consumption in the goldfish. Biol Bull 94:66–77. https://doi.org/10.2307/1538211
Gardner JL, Peters A, Kearney MR et al (2011) Declining body size: a third universal response to warming? Trends Ecol Evol 26:285–291
Gattuso J-P, Gao K, Lee K et al (2010) Guide to best practices for ocean acidification research and data reporting. Mar Chem 171:36–43
Gervais CR, Nay TJ, Renshaw G et al (2018) Too hot to handle? Using movement to alleviate effects of elevated temperatures in a benthic elasmobranch Hemiscyllium Ocellatum. Mar Biol 165:162. https://doi.org/10.1007/s00227-018-3427-7
Gervais CR, Huveneers C, Rummer JL, Brown C (2021) Population variation in the thermal response to climate change reveals differing sensitivity in a benthic shark. Glob Change Biol 27:108–120
Green L, Jutfelt F (2014) Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol Lett 10:20140538. https://doi.org/10.1098/rsbl.2014.0538
Hamilton TJ, Holcombe A, Tresguerres M (2014) CO2-induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proc Royal Soc B: Biol Sci 281(1775):20132509
Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3:1016–1030. https://doi.org/10.1002/ece3.516
Hawkins AJS, Day AJ (1996) The metabolic basis of genetic differences in growth efficiency among marine animals. J Exp Mar Biol Ecol 203:93–115. https://doi.org/10.1016/0022-0981(96)02572-5
Hein AM, Keirsted KJ (2012) The rising cost of warming waters: effects of temperature on the cost of swimming in fishes. Biol Let 8:266–269. https://doi.org/10.1098/rsbl.2011.0885
Heinrich DD, Watson S-A, Rummer JL et al (2016) Foraging behaviour of the epaulette shark Hemiscyllium ocellatum is not affected by elevated CO2. ICES J Mar Sci 73:633–640
Hight BV, Lowe CG (2007) Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: evidence for behavioral thermoregulation? J Exp Mar Biol Ecol 352:114–128
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485. https://doi.org/10.1038/nature09670
Intergovernmental Panel on Climate Change (2015) Climate change 2014: mitigation of climate change. Cambridge University Press
Intergovernmental Panel on Climate Change (2018) Global warming of 1.5 °C: an IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Intergovernmental Panel on Climate Change
Jain KE, Farrell AP (2003) Influence of seasonal temperature on the repeat swimming performance of rainbow trout Oncorhynchus mykiss. J Exp Biol 206:3569–3579. https://doi.org/10.1242/jeb.00588
Jayne BC, Lauder GV (1994) How swimming fish use slow and fast muscle fibers: implications for models of vertebrate muscle recruitment. J Comp Physiol A 175:123–131. https://doi.org/10.1007/BF00217443
Johnson T, Bennett A (1995) The thermal acclimation of burst escape performance in fish: an integrated study of molecular and cellular physiology and organismal performance. J Exp Biol 198:2165–2175. https://doi.org/10.1242/jeb.198.10.2165
Johnson KM, Hofmann GE (2020) Combined stress of ocean acidification and warming influence survival and drives differential gene expression patterns in the Antarctic pteropod, Limacina helicina antarctica. Conserv Physiol. https://doi.org/10.1093/conphys/coaa013
Johnson MS, Kraver DW, Renshaw GMC, Rummer JL (2016) Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv Physiol 4(1):cow003. https://doi.org/10.1093/conphys/cow003
Johnston IA, Johnson TP, Battram JC (1991) Low temperature limits burst swimming performance in Antarctic fish. In: di Prisco Guido, Maresca Bruno, Tota Bruno (eds) Biology of Antarctic Fish. Springer Berlin Heidelberg, Berlin, pp 179–190. https://doi.org/10.1007/978-3-642-76217-8_12
Jung H, Baek M, D’Elia KP et al (2018) The ancient origins of neural substrates for land walking. Cell 172:667–682
Kneebone J, Sulikowski J, Knotek R et al (2020) Using conventional and pop-up satellite transmitting tags to assess the horizontal movements and habitat use of thorny skate (Amblyraja radiata) in the Gulf of Maine. ICES J Mar Sci 77:2790–2803. https://doi.org/10.1093/icesjms/fsaa149
Koester DM, Spirito CP (2003) Punting: an unusual mode of locomotion in the little skate, Leucoraja erinacea (Chondrichthyes: Rajidae). Copeia 2003:553–561
Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13:1419–1434
Kroeker KJ, Kordas RL, Crim R et al (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896
Lauder GV (2015) Fish Locomotion: recent advances and new directions. Ann Rev Mar Sci 7:521–545. https://doi.org/10.1146/annurev-marine-010814-015614
Lauder GV, Di Santo V (2015) Swimming mechanics and energetics of elasmobranch fishes. In: Shadwick RE, Farrell AP, Brauner CJ (eds) Fish physiology. Academic Press, pp 219–53
Lawson CL, Halsey LG, Hays GC et al (2019) Powering ocean giants: the energetics of shark and ray megafauna. Trends Ecol Evol 34:1009–1021. https://doi.org/10.1016/j.tree.2019.07.001
Laxson CJ, Condon NE, Drazen JC, Yancey PH (2011) Decreasing urea∶ trimethylamine N-oxide ratios with depth in chondrichthyes: a physiological depth limit? Physiol Biochem Zool 84:494–505
Lear KO, Whitney NM, Morgan DL et al (2019) Thermal performance responses in free-ranging elasmobranchs depend on habitat use and body size. Oecologia 191:829–842. https://doi.org/10.1007/s00442-019-04547-1
Leonard JB, Summers AP, Koob TJ (1999) Metabolic rate of embryonic little skate, Raja erinacea (Chondrichthyes: Batoidea): the cost of active pumping. J Exp Zool 283:13–18
Lindsey CC (1978) Form, function, and locomotory habits in fish. Locomotion. Elsevier, pp 1–100. https://doi.org/10.1016/S1546-5098(08)60163-6
Lyon J, Ryan T, Scroggie M (2008) Effects of temperature on the fast-start swimming performance of an Australian freshwater fish. Ecol Freshw Fish 17:184–188
Macesic LJ, Summers AP (2012) Flexural stiffness and composition of the batoid propterygium as predictors of punting ability. J Exp Biol 215:2003–2012
Manes S, Costello MJ, Beckett H et al (2021) Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol Conserv 257:109070
Mavor TP, Bisagni JJ (2001) Seasonal variability of sea-surface temperature fronts on Georges Bank. Deep Sea Res Part II Top Stud Oceanogr 48:215–243
McDonnell LH, Chapman LJ (2016) Effects of thermal increase on aerobic capacity and swim performance in a tropical inland fish. Comp Biochem Physiol A Mol Integr Physiol 199:62–70
McKenzie DJ, Zhang Y, Eliason EJ, Schulte PM, Claireaux G, Blasco FR, Nati JJH, Farrell AP (2020) Intraspecific variation in tolerance of warming in fishes. J Fish Biol 98(6):1536–1555. https://doi.org/10.1111/jfb.14620
Meese EN, Lowe CG (2019) Finding a resting place: how environmental conditions influence the habitat selection of resting batoids. Bull South Calif Acad Sci 118:87–101. https://doi.org/10.3160/0038-3872-118.2.87
Melzner F, Gutowska M, Langenbuch M et al (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6:2313–2331
Mirasole A, Scopelliti G, Tramati C et al (2020) Evidences on alterations in skeleton composition and mineralization in a site-attached fish under naturally acidified conditions in a shallow CO2 vent. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.143309
Munday PL (2014) Transgenerational acclimation of fishes to climate change and ocean acidification. F1000Prime Rep. https://doi.org/10.12703/P6-99
Munday PL, Crawley NE, Nilsson GE (2009) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388:235–242
Nay TJ, Longbottom RJ, Gervais CR et al (2021) Regulate or tolerate: thermal strategy of a coral reef flat resident, the epaulette shark, Hemiscyllium ocellatum. J Fish Biol 98:723–732
Nelson GJ (1970) Outline of a theory of comparative biology. Syst Zool 19:373–384. https://doi.org/10.2307/2412278
Oeffner J, Lauder GV (2012) The hydrodynamic function of shark skin and two biomimetic applications. J Exp Biol 215:785–795
Papastamatiou YP, Watanabe YY, Bradley D et al (2015) Drivers of daily routines in an ectothermic marine predator: hunt warm, rest warmer? PLoS One 10:e0127807
Papastamatiou YP, Iosilevskii G, Di Santo V et al (2021) Sharks surf the slope: current updrafts reduce energy expenditure for aggregating marine predators. J Anim Ecol 90:2302–2314. https://doi.org/10.1111/1365-2656.13536
Parmesan C, Ryrholm N, Stefanescu C et al (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Parsons G, Carlson J (1998) Physiological and behavioral responses to hypoxia in the bonnethead shark, Sphyrna tiburo: routine swimming and respiratory regulation. Fish Physiol Biochem 19:189–196
Pauly Daniel (2021) The gill-oxygen limitation theory (GOLT) and its critics. Sci Adv. https://doi.org/10.1126/sciadv.abc6050
Payne NL, Meyer CG, Smith JA et al (2018) Combining abundance and performance data reveals how temperature regulates coastal occurrences and activity of a roaming apex predator. Glob Change Biol 24:1884–1893. https://doi.org/10.1111/gcb.14088
PerryLow ALJP, Ellis RJ, Reynolds DJ (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915. https://doi.org/10.1126/science.1111322
Pimentel MS, Faleiro F, Dionisio G et al (2014) Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J Exp Biol 217:2062–2070. https://doi.org/10.1242/jeb.092635
Pimentel MS, Faleiro F, Marques T et al (2016) Foraging behaviour, swimming performance and malformations of early stages of commercially important fishes under ocean acidification and warming. Clim Change 137:495–509. https://doi.org/10.1007/s10584-016-1682-5
Pistevos JCA, Nagelkerken I, Rossi T et al (2015) Ocean acidification and global warming impair shark hunting behaviour and growth. Sci Rep 5:16293. https://doi.org/10.1038/srep16293
Pistevos JCA, Nagelkerken I, Rossi T, Connell SD (2017) Antagonistic effects of ocean acidification and warming on hunting sharks. Oikos. https://doi.org/10.1111/oik.03182
Porter ME, Long JH (2010) Vertebrae in compression: mechanical behavior of arches and centra in the gray smooth-hound shark (Mustelus californicus). J Morphol 271:366–375
Porter ME, Ruddy BT, Kajiura SM (2020) Volitional swimming kinematics of blacktip sharks, Carcharhinus limbatus in the wild. Drones 4:78. https://doi.org/10.3390/drones4040078
Pough FH (1988) Evolutionary physiology: new directions in ecological physiology. Science 240:1349–1351. https://doi.org/10.1126/science.240.4857.1349
Powell M, Jones M, Lijalad M (2009) Effects of skeletal deformities on swimming performance and recovery from exhaustive exercise in triploid Atlantic salmon. Dis Aquat Org 85:59–66. https://doi.org/10.3354/dao02056
Priede IG, Froese R, Bailey DM et al (2006) The absence of sharks from abyssal regions of the world’s oceans. Proc R Soc B 273:1435–1441. https://doi.org/10.1098/rspb.2005.3461
Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Watson A (2005) Ocean acidification due to increasing atmospheric carbon dioxide. The Royal Society
Riebesell U, Fabry VJ, Hansson L, Gattuso JP (2011). Guide to best practices for ocean acidification research and data reporting. Office for official publications of the European communities
Roche DG, Binning SA, Bosiger Y et al (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216:2103–2110
Rosa R, Baptista M, Lopes VM et al (2014) Early-life exposure to climate change impairs tropical shark survival. Proc R Soc B 281:20141738. https://doi.org/10.1098/rspb.2014.1738
Rosa R, Rummer JL, Munday PL (2017a) Biological responses of sharks to ocean acidification. Biol Lett 13:20160796. https://doi.org/10.1098/rsbl.2016.0796
Rosa R, Rummer JL, Munday PL (2017b) Biological responses of sharks to ocean acidification. Biol Lett 13:20160796
Rosenberger LJ (2001) Pectoral fin locomotion in batoid fishes: undulation versus oscillation. J Exp Biol 204:379–394
Rosenberger LJ, Westneat MW (1999) Functional morphology of undulatory pectoral fin locomotion in the stingray Taeniura lymma (Chondrichthyes: Dasyatidae). J Exp Biol 202:3523–3539
Rummer JL, Stecyk JAW, Couturier CS, Watson S-A, Nilsson GE, Munday PL (2013) Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv Physiol 1(1):cot023. https://doi.org/10.1093/conphys/cot023
Rummer JL, Couturier CS, Stecyk JAW et al (2014) Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Change Biol 20:1055–1066. https://doi.org/10.1111/gcb.12455
Rummer JL, Binning SA, Roche DG, Johansen JL (2016) Methods matter: considering locomotory mode and respirometry technique when estimating metabolic rates of fishes. Conserv Physiol 4(1):cow008. https://doi.org/10.1093/conphys/cow008
Ryu T, Veilleux HD, Donelson JM et al (2018) The epigenetic landscape of transgenerational acclimation to ocean warming. Nat Clim Change 8:504–509. https://doi.org/10.1038/s41558-018-0159-0
Saadat M, Fish FE, Domel AG et al (2017) On the rules for aquatic locomotion. Phys Rev Fluids 2:083102
Saba VS, Griffies SM, Anderson WG et al (2016) Enhanced warming of the Northwest Atlantic Ocean under climate change. J Geophys Res Oceans 121:118–132. https://doi.org/10.1002/2015JC011346
Sassi A, Annabi A, Kessabi K et al (2010) Influence of high temperature on cadmium-induced skeletal deformities in juvenile mosquitofish (Gambusia affinis). Fish Physiol Biochem 36:403–409. https://doi.org/10.1007/s10695-009-9307-9
Schade FM, Clemmesen C, Wegner KM (2014) Within-and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus). Mar Biol 161:1667–1676
Schlegel P, Havenhand JN, Gillings MR, Williamson JE (2012) Individual variability in reproductive success determines winners and losers under ocean acidification: a case study with sea urchins. PloS One 7:53118
Schwieterman C, Anderson et al (2019) Combined effects of acute temperature change and elevated pCO2 on the metabolic rates and hypoxia tolerances of Clearnose Skate (Rostaraja eglanteria), Summer Flounder (Paralichthys dentatus), and Thorny Skate (Amblyraja radiata). Biology 8:56. https://doi.org/10.3390/biology8030056
Seibel BA, Maas AE, Dierssen HM (2012) Energetic plasticity underlies a variable response to ocean acidification in the pteropod Limacina helicina antarctica. PLoS One 7:e30464
Sfakiotakis M, Lane DM, Davies JBC (1999) Review of fish swimming modes for aquatic locomotion. IEEE J Ocean Eng 24:237–252
Shama LNS, Strobel A, Mark FC, Wegner KM (2014) Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct Ecol 28:1482–1493. https://doi.org/10.1111/1365-2435.12280
Silva-Garay L, Lowe CG (2021) Effects of temperature and body-mass on the standard metabolic rates of the round stingray, Urobatis halleri (Cooper, 1863). J Exp Mar Biol Ecol 540:151564. https://doi.org/10.1016/j.jembe.2021.151564
Sims DW (2010) Swimming behaviour and energetics of free-ranging sharks: New directions in movement analysis. In: Domenici P, Kapoor BG (eds) Fish locomotion, 1st edn. CRC Press, pp 407–435
Sims DW, Wearmouth VJ, Southall EJ et al (2006) Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. J Anim Ecol 75:176–190
Somero G (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers.’ J Exp Biol 213:912–920
Speed CW, Meekan MG, Field IC et al (2012) Heat-seeking sharks: support for behavioural thermoregulation in reef sharks. Mar Ecol Prog Ser 463:231–244
Stebbing ARD, Turk SMT, Wheeler A, Clarke KR (2002) Immigration of southern fish species to south-west England linked to warming of the North Atlantic (1960–2001). J Mar Biol Assoc 82:177–180. https://doi.org/10.1017/S0025315402005325
Steinhausen M, Sandblom E, Eliason E et al (2008) The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J Exp Biol 211:3915–3926
Takle H, Baeverfjord G, Lunde M et al (2005) The effect of heat and cold exposure on HSP70 expression and development of deformities during embryogenesis of Atlantic salmon (Salmo salar). Aquaculture 249:515–524. https://doi.org/10.1016/j.aquaculture.2005.04.043
Vedor M, Queiroz N, Mucientes G, Couto A, da Costa I, dos Santos A, Vandeperre F, Fontes J, Afonso P, Rosa R, Humphries NE, Sims DW (2021) eLife 10:e62508. https://doi.org/10.7554/eLife.62508
Veilleux HD, Ryu T, Donelson JM et al (2015) Molecular processes of transgenerational acclimation to a warming ocean. Nat Clim Change 5:1074–1078. https://doi.org/10.1038/nclimate2724
von Herbing IH (2002) Effects of temperature on larval fish swimming performance: the importance of physics to physiology. J Fish Biol 61:865–876. https://doi.org/10.1111/j.1095-8649.2002.tb01848.x
Wallman HL, Bennett WA (2006) Effects of parturition and feeding on thermal preference of Atlantic stingray, Dasyatis sabina (Lesueur). Environ Biol Fishes 75:259–267
Wang X, Song L, Chen Y et al (2017) Impact of ocean acidification on the early development and escape behavior of marine medaka (Oryzias melastigma). Mar Environ Res 131:10–18. https://doi.org/10.1016/j.marenvres.2017.09.001
Watson S-A, Allan BJM, McQueen DE et al (2018) Ocean warming has a greater effect than acidification on the early life history development and swimming performance of a large circumglobal pelagic fish. Glob Change Biol 24:4368–4385. https://doi.org/10.1111/gcb.14290
Webb PW (1994) The biology of fish swimming. In: Maddock L, Bone Q, Rayner JMV (eds) The mechanics and physiology of animal swimming. Cambridge University Press, pp 45–62. https://doi.org/10.1017/CBO9780511983641.005
Wellington GM, Victor BC (1989) Planktonic larval duration of one hundred species of Pacific and Atlantic damselfishes (Pomacentridae). Mar Biol. https://doi.org/10.1007/BF00541659
Wen L, Weaver JC, Lauder GV (2014) Biomimetic shark skin: design, fabrication and hydrodynamic function. J Exp Biol 217:1656–1666
Wen L, Ren Z, Di Santo V et al (2018) Understanding fish linear acceleration using an undulatory biorobotic model with soft fluidic elastomer actuated morphing median fins. Soft Robot 5:375–388. https://doi.org/10.1089/soro.2017.0085
Wheeler CR, Gervais CR, Johnson MS et al (2020) Anthropogenic stressors influence reproduction and development in elasmobranch fishes. Rev Fish Biol Fish 30:373–386
Wheeler CR, Rummer JL, Bailey B et al (2021) Future thermal regimes for epaulette sharks (Hemiscyllium ocellatum): growth and metabolic performance cease to be optimal. Sci Rep 11:1–12
Whitney NM, Papastamatiou YP, Holland KN, Lowe CG (2007) Use of an acceleration data logger to measure diel activity patterns in captive whitetip reef sharks, Triaenodon obesus. Aquat Living Resour 20:299–305. https://doi.org/10.1051/alr:2008006
Wilga CA, Lauder GV (2004) Biomechanics of locomotion in sharks, rays, and chimeras. Biol Sharks Relat 5:139–164
Wilson RS, Franklin CE, Davison W, Kraft P (2001) Stenotherms at sub-zero temperatures: thermal dependence of swimming performance in Antarctic fish. J Comp Physiol B 171:263–269. https://doi.org/10.1007/s003600000172
Witt WC, Wen L, Lauder GV (2015) Hydrodynamics of c-start escape responses of fish as studied with simple physical models. Integr Comp Biol 55:728–739. https://doi.org/10.1093/icb/icv016
Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3:995–1001. https://doi.org/10.1038/nclimate1982
Yancey P, Clark M, Hand S et al (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214. https://doi.org/10.1126/science.7112124
Yancey PH, Gerringer ME, Drazen JC et al (2014) Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc Natl Acad Sci 111:4461–4465
Yancey PH, Speers-Roesch B, Atchinson S et al (2018) Osmolyte adjustments as a pressure adaptation in deep-sea chondrichthyan fishes: an intraspecific test in arctic skates (Amblyraja hyperborea) along a depth gradient. Physiol Biochem Zool 91:788–796
Ytteborg E, Torgersen J, Baeverfjord G, Takle H (2010) Morphological and molecular characterization of developing vertebral fusions using a teleost model. BMC Physiol 10:13. https://doi.org/10.1186/1472-6793-10-13
Acknowledgements
The authors thank two anonymous reviewers for helpful suggestions on previous versions of this manuscript.
Funding
Open access funding provided by Stockholm University.
Author information
Authors and Affiliations
Contributions
MV and VDS conceived the paper, MV wrote the first draft of the manuscript, VDS wrote and revised successive versions of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vilmar, M., Di Santo, V. Swimming performance of sharks and rays under climate change. Rev Fish Biol Fisheries 32, 765–781 (2022). https://doi.org/10.1007/s11160-022-09706-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09706-x