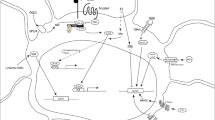
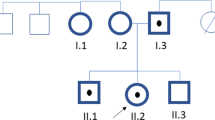
Abstract
A dozen years ago the identification of causal mutations in the low-density lipoprotein receptor-related protein 5 (LRP5) gene involved in two rare bone disorders propelled research in the bone field in totally new directions. Since then, there have been an explosion in the number of reports that highlight the role of the Wnt/β-catenin pathway in the regulation of bone homeostasis. In this review we discuss some of the most recent reports (in the past 2 years) highlighting the involvement of the members of the LRP family (LRP5, LRP6, LRP4, and more recently LRP8) in the maintenance of bone and their implications in bone diseases. These reports include records of new single nucleotides polymorphisms (SNPs) and haplotypes that suggest variants in these genes can contribute to subtle variation in bone traits to mutations that give rise to extreme bone phenotypes. All of these serve to further support and reinforce the importance of this tightly regulated pathway in bone. Furthermore, we discuss provocative reports suggesting novel approaches through inhibitors of this pathway to treat rarer diseases such as Osteoporosis-Pseudoglioma Syndrome (OPPG), Osteogenesis Imperfecta (OI), and Sclerosteosis/Van Buchem disease. It is hoped that by understanding the role of each component of the pathway and their involvement in bone diseases that this knowledge will allow us to develop new, more effective therapeutic approaches for more common diseases such as post-menopausal osteoporosis, osteoarthritis, and rheumatoid arthritis as well as these rarer bone diseases.

Similar content being viewed by others
References
Gong Y, Vikkula M, Boon L, Liu J, Beighton P, Ramesar R, et al. Osteoporosis-pseudoglioma syndrome, a disorder affecting skeletal strength and vision, is assigned to chromosome region 11q12-13. Am J Hum Genet. 1996;59:146–51.
Johnson ML, Gong G, Kimberling W, Recker SM, Kimmel DB, Recker RB. Linkage of a gene causing high bone mass to human chromosome 11 (11q12-13). Am J Hum Genet. 1997;60(6):1326–32.
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23.
Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–9. doi:10.1086/338450.
Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–5. doi:10.1038/35035117.
Balemans W, Devogelaer J-P, Cleiren E, Piters E, Caussin E, Van Hul W. Novel LRP5 missense mutation in a patient with a high bone mass phenotype results in decreased DKK1-mediated inhibition of Wnt signaling*. J Bone Miner Res. 2007;22(5):708–16. doi:10.1359/jbmr.070211.
Barros E, Dias da Silva M, Kunii I, Hauache O, Lazaretti-Castro M. A novel mutation in the LRP5 gene is associated with osteoporosis-pseudoglioma syndrome. Osteoporos Int. 2007;18(7):1017–8. doi:10.1007/s00198-007-0360-x.
Marques-Pinheiro A, Levasseur R, Cormier C, Bonneau J, Boileau C, Varret M, et al. Novel LRP5 gene mutation in a patient with osteoporosis-pseudoglioma syndrome. Joint Bone Spine. 2010;77(2):151–3.
Narumi S, Numakura C, Shiihara T, Seiwa C, Nozaki Y, Yamagata T, et al. Various types of LRP5 mutations in four patients with osteoporosis-pseudoglioma syndrome: Identification of a 7.2-kb microdeletion using oligonucleotide tiling microarray. Am J Med Genet A. 2010;152A(1):133–40. doi:10.1002/ajmg.a.33177.
Okubo M, Horinishi A, Kim DH, Yamamoto TT, Murase T. Seven novel sequence variants in the human low density lipoprotein receptor related protein 5 (LRP5) gene. Hum Mutat. 2002;19:186–8.
Saarinen A, Valimaki VV, Valimaki MJ, Loyttyniemi E, Auro K, Uusen P, et al. The A1330V polymorhpism of the low-density lipoprotein receptor-related protein 5 gene (LRP5) associates with low peak bone mass in young healthy men. Bone. 2007;40:1006–12.
Streeten EA, McBride DJ, Lodge AL, Pollin TI, Stinchcomb DG, Agarwala R, et al. Reduced incidence of hip fracture in the Old Order Amish. J Bone Miner Res. 2004;19(2):308–13. doi:10.1359/JBMR.0301223.
Streeten EA, Puffenberger E, Morton H, McBride D. Osteoporosis pseudoglioma syndrome: 4 siblings with a compound heterozygote LRP5 mutation. J Bone Miner Res. 2004;19 Suppl 1:S182.
Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72(3):763–71. doi:10.1086/368277.
Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359(2):222–9. doi:10.1016/j.ydbio.2011.08.020.
Joiner DM, Less KD, Van Wieren EM, Hess D, Williams BO. Heterozygosity for an inactivating mutation in low-density lipoprotein-related receptor 6 (Lrp6) increases osteoarthritis severity in mice after ligament and meniscus injury. Osteoarthr Cartil/OARS Osteoarthr Res Society. 2013;21(10):1576–85. doi:10.1016/j.joca.2013.05.019.
Riddle RC, Diegel CR, Leslie JM, Van Koevering KK, Faugere MC, Clemens TL, et al. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PLoS One. 2013;8(5), e63323. doi:10.1371/journal.pone.0063323.
Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS ONE. 2009;4(11), e7930.
Kumar J, Swanberg M, McGuigan F, Callreus M, Gerdhem P, Åkesson K. LRP4 association to bone properties and fracture and interaction with genes in the Wnt- and BMP signaling pathways. Bone. 2011;49(3):343–8.
Li Y, Pawlik B, Elcioglu N, Aglan M, Kayserili H, Yigit G, et al. LRP4 mutations alter Wnt/[beta]-catenin signaling and cause limb and kidney malformations in cenani-lenz syndrome. Am J Hum Genet. 2010;86(5):696–706.
Simon-Chazottes D, Tutois S, Kuehn M, Evans M, Bourgade F, Cook S, et al. Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics. 2006;87(5):673–7.
Experimental drug may change the treatment of osteoporosis. Romosozumab appears to increase bone mineral density and help rebuild the skeleton at the same time. DukeMedicine healthnews. 2014;20(4):7.
Costa AG, Bilezikian JP, Lewiecki EM. Update on romosozumab: a humanized monoclonal antibody to sclerostin. Expert Opin Biol Ther. 2014;14(5):697–707. doi:10.1517/14712598.2014.895808.
Evenepoel P, D’Haese P, Brandenburg V. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370(17):1664. doi:10.1056/NEJMc1402396#SA1.
McClung MR, Grauer A. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370(17):1664–5. doi:10.1056/NEJMc1402396.
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20. doi:10.1056/NEJMoa1305224.
McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29(4):935–43. doi:10.1002/jbmr.2092.
Recker R, Benson C, Matsumoto T, Bolognese M, Robins D, Alam J, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2014. doi:10.1002/jbmr.2351.
Montagnani A. Bone anabolics in osteoporosis: actuality and perspectives. World J Orthop. 2014;5(3):247–54. doi:10.5312/wjo.v5.i3.247.
Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev Dyn Off Publ Am Assoc Anatomists. 2010;239(1):102–14. doi:10.1002/dvdy.22181.
Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, et al. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun. 1998;248:879–88.
Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol. 2001;23(1):53–67. doi:10.1385/MN:23:1:53.
Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–7. doi:10.1038/nature04185.
Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–56.
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–804. doi:10.1093/emboj/16.13.3797.
Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, et al. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 2002;21(7):1733–42. doi:10.1093/emboj/21.7.1733.
Nusse R. Repression and activation. Trends in genetics. TIG. 1999;15(1):1–3.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7. doi:10.1074/jbc.M413274200.
Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274(27):19465–72.
Bao J, Zheng JJ, Wu D. The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci Signal. 2012;5(224), e22. doi:10.1126/scisignal.2003028.
Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-Catenin signalling. Nature. 2002;417:664–7.
Nakamura T, Aoki S, Kitajima K, Takahashi T, Matsumoto K, Nakamura T. Molecular cloning and characterization of Kremen, a novel kringle-containing transmembrane protein. Biochim Biophys Acta. 2001;1518(1–2):63–72.
Cselenyi CS, Lee E. Context-dependent activation or inhibition of Wnt-beta-catenin signaling by Kremen. Sci Signal. 2008;1(8), e10. doi:10.1126/stke.18pe10.
Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, et al. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn Off Publ Am Assoc Anat. 2006;235(3):681–90. doi:10.1002/dvdy.20681.
Surana R, Sikka S, Cai W, Shin EM, Warrier SR, Tan HJ, et al. Secreted frizzled related proteins: implications in cancers. Biochim Biophys Acta. 2014;1845(1):53–65. doi:10.1016/j.bbcan.2013.11.004.
Guidato S, Itasaki N. Wise retained in the endoplasmic reticulum inhibits Wnt signaling by reducing cell surface LRP6. Dev Biol. 2007;310(2):250–63. doi:10.1016/j.ydbio.2007.07.033.
Lintern KB, Guidato S, Rowe A, Saldanha JW, Itasaki N. Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J Biol Chem. 2009;284(34):23159–68. doi:10.1074/jbc.M109.025478.
Ellies DL, Economou A, Viviano B, Rey JP, Paine-Saunders S, Krumlauf R, et al. Wise regulates bone deposition through genetic interactions with Lrp5. PLoS One. 2014;9(5), e96257. doi:10.1371/journal.pone.0096257.
Balemans W, Piters E, Cleiren E, Ai M, Van Wesenbeeck L, Warman ML, et al. The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int. 2008;82(6):445–53. doi:10.1007/s00223-008-9130-9.
Boudin E, Steenackers E, de Freitas F, Nielsen TL, Andersen M, Brixen K, et al. A common LRP4 haplotype is associated with bone mineral density and hip geometry in men-data from the Odense Androgen Study (OAS). Bone. 2013;53(2):414–20. doi:10.1016/j.bone.2013.01.014.
Zhang J, Zhang X, Zhang L, Zhou F, van Dinther M, Ten Dijke P. LRP8 mediates Wnt/beta-catenin signaling and controls osteoblast differentiation. J Bone Miner Res. 2012;27(10):2065–74. doi:10.1002/jbmr.1661.
Kato M, Patel MS, Levasseur R, Lobov I, Chang BH-J, Glass DA, et al. Cbfa 1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14.
Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18(6):960–74. doi:10.1359/jbmr.2003.18.6.960.
Niziolek PJ, Warman ML, Robling AG. Mechanotransduction in bone tissue: the A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone. 2012;51(3):459–65. doi:10.1016/j.bone.2012.05.023.
Javaheri B, Sunters A, Zaman G, Suswillo RF, Saxon LK, Lanyon LE, et al. Lrp5 is not required for the proliferative response of osteoblasts to strain but regulates proliferation and apoptosis in a cell autonomous manner. PLoS One. 2012;7(5), e35726. doi:10.1371/journal.pone.0035726.
Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young’s modulus and responsiveness to the mechanical loading. Bone. 2013;54(1):35–43. doi:10.1016/j.bone.2013.01.033.
Falcon-Ramirez E, Casas-Avila L, Cerda-Flores RM, Castro-Hernandez C, Rubio-Lightbourn J, Velazquez-Cruz R, et al. Association of LRP5 haplotypes with osteoporosis in Mexican women. Mol Biol Rep. 2013;40(3):2705–10. doi:10.1007/s11033-012-2357-6.
Sassi R, Sahli H, Souissi C, El Mahmoudi H, Zouari B, ElGaaied ABA, et al. Association of LRP5 genotypes with osteoporosis in Tunisian post-menopausal women. BMC Musculoskelet Disord. 2014;15:144. doi:10.1186/1471-2474-15-144.
Park SE, Oh KW, Lee WY, Baek KH, Yoon KH, Son HY et al. Association of osteoporosis susceptibility genes with bone mineral density and bone metabolism related markers in Koreans: The Chungju Metabolic Disease Cohort (CMC) study. Endocr J. 2014;61(11):1069–78
Campos-Obando N, Oei L, Hoefsloot LH, Kiewiet RM, Klaver CC, Simon ME, et al. Osteoporotic vertebral fractures during pregnancy: be aware of a potential underlying genetic cause. J Clin Endocrinol Metab. 2014;99(4):1107–11. doi:10.1210/jc.2013-3238.
Chapman K, Mustafa Z, Dowling B, Southam L, Carr A, Loughlin J. Finer linkage mapping of primary hip osteoarthritis susceptibility on chromosome 11q in a cohort of affected female sibling pairs. Arthritis Rheum. 2002;46(7):1780–3. doi:10.1002/art.10412.
Chapman K, Mustafa Z, Irven C, Carr AJ, Clipsham K, Smith A, et al. Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet. 1999;65(1):167–74. doi:10.1086/302465.
Shi XW, Guo X, Lv AL, Kang L, Zhou YL, Zhang YZ, et al. Heritability estimates and linkage analysis of 23 short tandem repeat loci on chromosomes 2, 11, and 12 in an endemic osteochondropathy in China. Scand J Rheumatol. 2010;39(3):259–65. doi:10.3109/03009740903270599.
Smith AJ, Gidley J, Sandy JR, Perry MJ, Elson CJ, Kirwan JR, et al. Haplotypes of the low-density lipoprotein receptor-related protein 5 (LRP5) gene: are they a risk factor in osteoarthritis? Osteoarthr Cartil/OARS Osteoarthr Res Soc. 2005;13(7):608–13. doi:10.1016/j.joca.2005.01.008.
Yerges-Armstrong LM, Yau MS, Liu Y, Krishnan S, Renner JB, Eaton CB, et al. Association analysis of BMD-associated SNPs with knee osteoarthritis. J Bone Miner Res. 2014;29(6):1373–9. doi:10.1002/jbmr.2160.
Lodewyckx L, Luyten FP, Lories RJ. Genetic deletion of low-density lipoprotein receptor-related protein 5 increases cartilage degradation in instability-induced osteoarthritis. Rheumatology (Oxford). 2012;51(11):1973–8. doi:10.1093/rheumatology/kes178.
Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15(5):426–39.
Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25(10):2069–78. doi:10.1016/j.cellsig.2013.04.002.
de Rooy DP, Yeremenko NG, Wilson AG, Knevel R, Lindqvist E, Saxne T, et al. Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2013;72(5):769–75. doi:10.1136/annrheumdis-2012-202184.
Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2013. doi:10.1002/jcph.239.
Chang MK, Kramer I, Keller H, Gooi JH, Collett C, Jenkins D, et al. Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity. J Bone Miner Res. 2014;29(1):29–42. doi:10.1002/jbmr.2059.
Kedlaya R, Veera S, Horan DJ, Moss RE, Ayturk UM, Jacobsen CM, et al. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci Transl Med. 2013;5(211):211ra158. doi:10.1126/scitranslmed.3006627.
Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res. 2014;29(10):2297–306. doi:10.1002/jbmr.2198.
Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone. 2014;66:182–8. doi:10.1016/j.bone.2014.06.015.
Lindahl K, Langdahl B, Ljunggren O, Kindmark A. Treatment of osteogenesis imperfecta in adults. Eur J Endocrinol/Eur Fed Endocr Soc. 2014;171(2):R79–90. doi:10.1530/EJE-14-0017.
Ngan KK, Bowe J, Goodger N. The risk of bisphosphonate-related osteonecrosis of the jaw in children. A case report and literature review. Dent Updat. 2013;40(9):733–4. 9.
Christou J, Johnson AR, Hodgson TA. Bisphosphonate-related osteonecrosis of the jaws and its relevance to children--a review. Int J Paediatr Dent/Br Paedodontic Soc [and] Int Assoc Dent Child. 2013;23(5):330–7. doi:10.1111/ipd.12047.
Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013;28(1):73–80. doi:10.1002/jbmr.1717.
Sinder BP, White LE, Salemi JD, Ominsky MS, Caird MS, Marini JC, et al. Adult Brtl/+ mouse model of osteogenesis imperfecta demonstrates anabolic response to sclerostin antibody treatment with increased bone mass and strength. Osteoporos Int. 2014;25(8):2097–107. doi:10.1007/s00198-014-2737-y.
Acknowledgments
This work is funded by grants from the NIH NIAMS RO1 AR053949 and RC2 AR058962, NIA P01 AG039355, a grant from the UMKC CEMT through the Missouri Life Science Research Board, and a University of Missouri Research Board Faculty Grant.
Conflict of Interest Statement
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lara-Castillo, N., Johnson, M.L. LRP receptor family member associated bone disease. Rev Endocr Metab Disord 16, 141–148 (2015). https://doi.org/10.1007/s11154-015-9315-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-015-9315-2