Abstract
Summary
We present a family with a rare mutation of the LRP6 gene and for the first time provide evidence for its association with low bone mineral density.
Introduction
The Wnt pathway plays a critical role in bone homeostasis. Pathogenic variants of the Wnt co-receptor LRP6 have been associated with abnormal skeletal phenotypes or increased risk of cardiovascular events.
Patient and methods
Here we report an index premenopausal patient and her family carrying a rare missense LRP6 pathogenic variant (rs141212743; 0.0002 frequency among Europeans). This variant has been previously associated with metabolic syndrome and atherosclerosis, in the presence of normal bone mineral density. However, the LRP6 variant was associated with low bone mineral density in this family, without evidence for association with serum lipid levels or cardiovascular events.
Conclusion
Thus, this novel association shows that LRP6 pathogenic variants may be involved in some cases of early-onset osteoporosis, but the predominant effect, either skeletal or cardiovascular, may vary depending on the genetic background or other acquired factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The identification of some individuals carrying rare allelic variants of the LRP5 gene and a markedly abnormal bone mass led to the discovery of the important role of the Wnt pathway in skeletal homeostasis [1]. Wnt ligands comprise several molecules that bind to complex membrane receptors formed by frizzled proteins and LRP5/6 coreceptors and play a critical role in osteoblastogenesis [2]. It is well established that gain-of-function mutations of LRP5 are associated with a high bone mass phenotype, whereas inactivating mutations cause osteoporosis-pseudoglioma syndrome [3]. The role of LRP6 is less clear. A few patients with LRP6 pathogenic variants and skeletal or cardiovascular abnormalities have been reported [4, 5], but some preclinical studies suggest that LRP5 and LRP6 have redundant effects on bone [6]. Here, we report a female patient with early-onset osteoporosis and other family members. This is the first reported case of the association of this LRP6 variant with low bone mass. Patients’ characteristics suggest that the consequences of individual variants of LRP6 may be rather different across subjects with different genetic backgrounds or exposed to different acquired factors.
Case report and family data
A 45-year-old Caucasian premenopausal woman was sent to our outpatient bone clinic because of mild back pain and an X-ray suggestive of low bone density at the lumbar spine, but no fractures. She and her ancestors were born in Northern Spain. Her medical history was unremarkable. The physical exam was normal. A serum biochemical profile was normal, including total and ionized calcium, phosphorus, total alkaline phosphatase, intact PTH, TSH, protein electrophoresis, and 25-hydroxyvitamin D levels. Serum cholesterol was within the normal range (178 mg/dl, 4.6 mmol/l). The complete blood count was also normal. Serum P1NP was 50 ng/ml (reference range 19–102 ng/ml) and CTX was 0.225 ng/ml (reference range 0.170–0.760 ng/ml). Urinary calcium excretion was 242 mg/24 h. Bone mineral density (BMD) was measured at the spine and the hip by DXA (Horizon, Hologic). The Z-scores (this is, BMD expressed as standard deviations in comparison with normal individuals of the same sex and age) were − 2.8 at the lumbar spine, − 1.4 at the femoral neck, and − 1.2 at the total hip.
A custom panel of 130 genes (see supplementary information, Table S1) (SureSelect QXT, Agilent Technologies) related to bone disorders were sequenced (exons and 10 bp flanking intronic regions) and the bioinformatic analysis was performed using the Alissa platform from Agilent Technologies. It revealed a missense heterozygous pathogenic variant of the LRP6 gene (NC_000012.11:g.12334271C > T; NM_002336.2: c.1079G > A, p.Arg360His; rs141212743). No other pathogenic/likely pathogenic variants were detected.
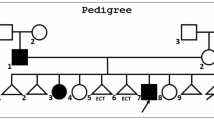
Family members were offered to be studied (Fig. 1). The presence or absence of the LRP6 variant was determined by Sanger sequencing. The father of the index patient and a 48-year-old brother carried the variant, whereas the mother, a maternal aunt, and another brother had normal genotype. The results of DXA scans of those six individuals are shown in Fig. 2. The mean Z-scores for the 3 heterozygous carriers of the variant were − 1.8 at the spine, − 1.4 at the femoral neck, and − 1.2 at the total hip. On the other hand, the mean Z-scores of the 3 non-carrier individuals were − 1.1 at the lumbar spine (p = 0.18 in comparison with carriers), 0.0 at the femoral neck (p = 0.003), and − 0.1, at the total hip (p = 0.0001). T-scores are shown in the supplementary information (Table S2). No individual suffered other disorders causing secondary osteoporosis, and vitamin D and other serum chemistries were within the normal limits.
Bone mineral density (expressed as Z-scores at the lumbar spine, femoral neck, and total hip) of the six family members. Carriers of the LRP6 variant are represented by dashed bars; noncarriers are represented by black bars. Individual keys correspond to those in pedigree in Fig. 1
Regarding cardiovascular data, the patient’s mother (I.2, 75-year-old, non-carrier) had no history of cardiovascular disease, her blood cholesterol level was 164 mg/dl (4.2 mmol/l). The patient’s father (I.3; 87-year-old, mutation carrier) had suffered coronary heart disease and a stroke; serum cholesterol was 138 mg/dl (3.6 mmol/l), while on statin therapy. A maternal aunt (I.1, 81-year-old, non-carrier) had coronary heart disease and cholesterol levels of 169 mg/dl (4.4 mmol/l) while on statins. The patient’s brothers included a 40-year-old man (II.3, non-carrier) and a 48-year-old-man (II.1, carrier) with cholesterol levels of 200 mg/dl and 203 mg/dl, respectively (5.2 mmol/l). Neither of them had known cardiovascular disease.
Discussion
The Arg360His LRP6 variant has been found in 1/4534 Europeans in the Genome Aggregation Database (gnomAD) (available at https://gnomad.broadinstitute.org/). It causes an amino acid change and is classified as likely pathogenic by the Varsome webtool [7]. The substituted residue lies in the second propeller domain, which is important for ligand release and recycling of the receptor and is highly conserved across species. The amino acid change (R to H) increases hydrophobicity and favors the β-sheet structure (see supplementary information, Fig. S1). Overall, 15 (BayesDel addAF, BayesDel noAF, EIGEN, EIGEN PC, FATHMM, FATHMM-MKL, FATHMM-XF, LRT, M-CA, MutationTaster, MVP, PrimateAI, PROVEAN, SIFT,SIFT4G) out of 17 bioinformatic predictors and all 4 meta-predictors (MetaLR, MetaSVM, MetaRMN, and REVEL) included in Varsome considered that it has a deleterious effect on protein function.
Wnt signaling is critical for skeletal homeostasis, and specifically for bone formation. However, the relative contribution of the Wnt co-receptors LRP5 and LRP6 is debated. Conditional deletion of LRP5 or LRP6 in cells of the osteoblastic lineage suggests that both receptors participate in Wnt signaling and that they carry out somewhat redundant functions and are involved in mechanotransduction [8]. However, other experiments suggested that some Wnt ligands may bind preferentially to either LRP5 or LRP6, the latter being most important in mediating Wnt3a signaling [9], as well as in maintaining the survival and proliferation of osteoblast precursors and transducing some effects of PTH on the bone [10].
A few LRP6 mutations have been reported in patients with osteoporosis. Sturnickel et al. [5] found 25 individuals with LRP5 allelic variants and 6 with LRP6 variants among 371 patients with early-onset osteoporosis, but none of the LRP6 variants were classified as damaging. However, no LRP6 mutations were found in other studies of osteoporosis in young people or among some familiar cases of osteoporosis [11,12,13,14]. Interestingly, recent reports show that, similarly to gain-of-function mutations of LRP5, some LRP6 activating mutations may result in generalized high bone mass or localized bone sclerosis [15, 16].
This Arg360His LRP6 variant was previously reported in an American patient with hyperlipidemia and premature atherosclerosis, but normal bone density [4]. On the contrary, in the family here reported, carriers of the mutation had low BMD, but there was no clear association with hyperlipidemia or cardiovascular events. However, since some individuals were in their forties, we do not know if they will eventually develop clinical atherosclerosis in the future.
In recent years, evidence for the role of LRP6 in cardiovascular homeostasis and disorders is accumulating [17]. However, it is to note that whereas in the previously reported family with the Arg360His mutation carriers had a cardiovascular phenotype, low bone mass seems to be the main phenotypic abnormality in the currently reported family. We cannot establish if those phenotypic differences are related to differences in the genetic background of the patients, or lifestyle and other environmental factors.
Regarding bone mass, we used Z-scores to allow comparing subjects of different sex and age. The difference in BMD between carriers and non-carriers appeared larger at the hip (1.1 to − 1.4 Z units) than at the spine (0.7 Z units). This is in line with the concept that Wnt signaling may be somewhat more important for attaining and maintaining cortical than trabecular bone mass, as suggested by some experimental data [18] and genetic association studies [19]. This concept is also supported by the thick bones found in patients with SOST gene mutations that result in an exaggerated activity of the Wnt pathway [20].
In summary, we report a family with low BMD in carriers of the Arg360His LRP6 mutation, without clear association with cardiovascular abnormalities. These results suggest that LRP6 mutations explain some cases of early-onset osteoporosis or cardiovascular disease, but the predominant phenotype may vary depending on other genetic and environmental factors.
References
Gong Y, Slee RB, Fukai N et al (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523
Houschyar KS, Tapking C, Borrelli MR et al (2019) Wnt pathway in bone repair and regeneration – what do we know so far. Front Cell Dev Biol 6:1–13. https://doi.org/10.3389/fcell.2018.00170
Balemans W, Van Hul W (2007) The genetics of low-density lipoprotein receptor-related protein 5 in bone: a story of extremes. Endocrinology 148:2622–2629
Singh R, Smith E, Fathzadeh M et al (2013) Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum Mutat 34:1221–1225. https://doi.org/10.1002/HUMU.22360
Stürznickel J, Rolvien T, Delsmann A et al (2021) Clinical phenotype and relevance of LRP5 and LRP6 variants in patients with early-onset osteoporosis (EOOP). J Bone Miner Res 36:271–282. https://doi.org/10.1002/JBMR.4197
Lim KE, Bullock WA, Horan DJ, et al (2021) Co-deletion of Lrp5 and Lrp6 in the skeleton severely diminishes bone gain from sclerostin antibody administration. Bone 143:115708. https://doi.org/10.1016/J.BONE.2020.115708
Kopanos C, Tsiolkas V, Kouris A et al (2019) VarSome: the human genomic variant search engine. Bioinformatics 35:1978–1980. https://doi.org/10.1093/BIOINFORMATICS/BTY897
Schumacher CA, Joiner DM, Less KD, et al (2016) Characterization of genetically engineered mouse models carrying Col2a1-cre-induced deletions of Lrp5 and/or Lrp6. Bone Res 4:15042. https://doi.org/10.1038/BONERES.2015.42
Sebastian A, Hum NR, Murugesh DK, et al (2017) Wnt co-receptors Lrp5 and Lrp6 differentially mediate Wnt3a signaling in osteoblasts. PLoS ONE 12. https://doi.org/10.1371/JOURNAL.PONE.0188264
Li C, Wang W, Xie L et al (2016) Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression. Ann N Y Acad Sci 1364:62–73. https://doi.org/10.1111/NYAS.12750
Franceschi R, Vincenzi M, Camilot M et al (2015) Idiopathic juvenile osteoporosis: clinical experience from a single centre and screening of LRP5 and LRP6 genes. Calcif Tissue Int 96:575–579. https://doi.org/10.1007/S00223-015-9983-7
Cilia C, Friggieri D, Vassallo J et al (2022) Whole genome sequencing unravels new genetic determinants of early-onset familial osteoporosis and low BMD in Malta. Genes (Basel) 13(2):204 https://doi.org/10.3390/GENES13020204
Teerlink CC, Jurynec MJ, Hernandez R et al (2021) A role for the MEGF6 gene in predisposition to osteoporosis. Ann Hum Genet 85:58–72. https://doi.org/10.1111/AHG.12408
Rocha-Braz MGM, França MM, Fernandes AM et al (2020) Comprehensive genetic analysis of 128 candidate genes in a cohort with idiopathic, severe, or familial osteoporosis. J Endocr Soc 4(12):1–13. https://doi.org/10.1210/JENDSO/BVAA148
Brance ML, Brun LR, Cóccaro NM et al (2020) High bone mass from mutation of low-density lipoprotein receptor-related protein 6 (LRP6). Bone 141: 115550. https://doi.org/10.1016/J.BONE.2020.115550
Pickering ME, Ltaief-Boudrigua A, Feurer E et al (2021) A new LRP6 variant and Camurati-Engelmann-like disease. Bone 143:115706. https://doi.org/10.1016/J.BONE.2020.115706
Kang S (2020) Low-density lipoprotein receptor-related protein 6-mediated signaling pathways and associated cardiovascular diseases: diagnostic and therapeutic opportunities. Hum Genet 139:447–459. https://doi.org/10.1007/S00439-020-02124-8
Wang F, Rummukainen P, Heino TJ, Kiviranta R (2021) Osteoblastic Wnt1 regulates periosteal bone formation in adult mice. Bone 143:115754. https://doi.org/10.1016/J.BONE.2020.115754
García-Ibarbia C, Pérez-Núñez MI, Olmos JM et al (2013) Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos Int 24:2449–2454. https://doi.org/10.1007/s00198-013-2302-0
Balemans W, Cleiren E, Siebers U et al (2005) A generalized skeletal hyperostosis in two siblings caused by a novel mutation in the SOST gene. Bone 36:943–947
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
N.P., data curation, original draft, review, and editing manuscript; A.V., data curation, review, and editing manuscript; J.L.H., conceptualization, review and editing manuscript; J.L.F-L, supervision, review and editing manuscript; J.A.R., conceptualization, formal analysis, supervision, writing—original draft and further review & editing.
Corresponding author
Ethics declarations
Consent to participate
Individuals signed informed consent for the genetic analysis and publication. All clinical and analytical procedures were performed as part of routine clinical care.
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Puente, N., Vega, A.I., Hernandez, J.L. et al. An LRP6 mutation (Arg360His) associated with low bone mineral density but not cardiovascular events in a Caucasian family. Osteoporos Int 33, 2445–2448 (2022). https://doi.org/10.1007/s00198-022-06494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06494-9