Abstract
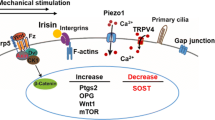
Skeletal loading is an important physiological regulator of bone mass. Theoretically, mechanical forces or administration of drugs that activate bone mechanosensors would be a novel treatment for osteoporotic disorders, particularly age-related osteoporosis and other bone loss caused by skeletal unloading. Uncertainty regarding the identity of the molecular targets that sense and transduce mechanical forces in bone, however, has limited the therapeutic exploitation of mechanosesning pathways to control bone mass. Recently, two evolutionally conserved mechanosensing pathways have been shown to function as “physical environment” sensors in cells of the osteoblasts lineage. Indeed, polycystin–1 (Pkd1, or PC1) and polycystin–2 (Pkd2, or PC2‚ or TRPP2), which form a flow sensing receptor channel complex, and TAZ (transcriptional coactivator with PDZ-binding motif, or WWTR1), which responds to the extracellular matrix microenvironment act in concert to reciprocally regulate osteoblastogenesis and adipogenesis through co-activating Runx2 and a co-repressing PPARγ activities. Interactions of polycystins and TAZ with other putative mechanosensing mechanism, such as primary cilia, integrins and hemichannels, may create multifaceted mechanosensing networks in bone. Moreover, modulation of polycystins and TAZ interactions identify novel molecular targets to develop small molecules that mimic the effects of mechanical loading on bone.



Similar content being viewed by others
References
Luu YK, Pessin JE, Judex S, Rubin J, Rubin CT. Mechanical signals as a non-invasive means to influence mesenchymal stem cell fate, promoting bone and suppressing the fat phenotype. Bonekey Osteovision. 2009;6(4):132–49. doi:10.1138/20090371.
Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149(12):6065–75. doi:10.1210/en.2008-0687.
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71.
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3(6):379–89. doi:10.1111/j.1474-9728.2004.00127.x.
Meunier P, Courpron P, Edouard C, Bernard J, Bringuier J, Vignon G. Physiological senile involution and pathological rarefaction of bone. Quantitative and comparative histological data. Clin Endocrinol Metab. 1973;2(2):239–56.
Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145(5):2421–32. doi:10.1210/en.2003-1156.
Hughes-Fulford M Signal transduction and mechanical stress. Sci STKE. 2004;2004(249):RE12.
Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328(3):751–5.
Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16.
Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88(1):104–12.
Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, et al. Mechanobiology of the skeleton. Sci Signal. 2009;2(68):pt3. doi:10.1126/scisignal.268pt3.
David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148(5):2553–62. doi:10.1210/en.2006-1704.
Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104(45):17879–84. doi:10.1073/pnas.0708467104.
Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279(29):30588–99. doi:10.1074/jbc.M313244200.
Du L, Fan H, Miao H, Zhao G, Hou Y. Extremely low frequency magnetic fields inhibit adipogenesis of human mesenchymal stem cells. Bioelectromagnetics. 2014;35(7):519–30. doi:10.1002/bem.21873.
Bonewald L Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3:7–15.
Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–15. doi:10.1016/j.bone.2007.12.224.
Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118–25. doi:10.1007/s11914-012-0105-4.
Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: Master Orchestrators of Bone. Calcif Tissue Int. 2013. doi:10.1007/s00223-013-9790-y.
Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: Implications for the study of mechanosensation in bone. Bone. 2010;47(5):872–81. doi:10.1016/j.bone.2010.08.007.
Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27(3):563–74. doi:10.1002/jbmr.1474.
Jing D, Lu XL, Luo E, Sajda P, Leong PL, Guo XE. Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid flow. Bone. 2013;53(2):531–40. doi:10.1016/j.bone.2013.01.008.
Galli C, Passeri G, Macaluso GM. Osteocytes and WNT: the mechanical control of bone formation. J Dent Res. 2010;89(4):331–43. doi:10.1177/0022034510363963.
Tatsumi S, Ishii K, Amizuka N, Li MQ, Kobayashi T, Kohno K, et al. Targeted ablation of Osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–75. doi:10.1016/j.cmet.2007.05.001.
Rochefort GY, Pallu S, Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int. 2010;21(9):1457–69. doi:10.1007/s00198-010-1194-5.
Santos A, Bakker AD, Klein-Nulend J. The role of osteocytes in bone mechanotransduction. Osteoporos Int. 2009;20(6):1027–31. doi:10.1007/s00198-009-0858-5.
Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28(2):145–9.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell... And more. Endocr Rev. 2013;34(5):658–90. doi:10.1210/er.2012-1026.
Burger EH, Klein-Nulend J, Smit TH. Strain-derived canalicular fluid flow regulates osteoclast activity in a remodelling osteon–a proposal. J Biomech. 2003;36(10):1453–9.
Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50(5):1115–22. doi:10.1016/j.bone.2012.01.025.
Bonewald LF. The amazing osteocyte. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(2):229–38. doi:10.1002/jbmr.320.
Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3(10):7–15.
Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53(Suppl 1):S102–6 discussion S6-7.
Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res Off J Am Soc Bone Miner Res. 2002;17(11):2068–79. doi:10.1359/jbmr.2002.17.11.2068.
Ehrlich PJ, Noble BS, Jessop HL, Stevens HY, Mosley JR, Lanyon LE. The effect of in vivo mechanical loading on estrogen receptor alpha expression in rat ulnar osteocytes. J Bone Miner Res. 2002;17(9):1646–55. doi:10.1359/jbmr.2002.17.9.1646.
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–17. doi:10.1016/j.bone.2011.10.025.
Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281(41):30884–95.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi:10.1038/nature10137.
Varelas X The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–26. doi:10.1242/dev.102376.
Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol Quant Biosci Nano Macro. 2010;2(9):435–42. doi:10.1039/c0ib00034e.
Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119(Pt 3):508–18. doi:10.1242/jcs.02760.
Siller-Jackson AJ, Burra S, Gu S, Harris SE, Boenwald LF, Sprague E, et al. The role of alpha5 integrin as a mechanosensor in the regulation of connexin 43 hemichaneel release of prostaglandin in response to mechanical stress. J Bone Miner Res. 2006;21(Suppl 1):S72.
Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res. 2008;23(6):879–86. doi:10.1359/jbmr.080222.
Loiselle AE, Paul EM, Lewis GS, Donahue HJ. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J Orthop Res. 2013;31(1):147–54. doi:10.1002/jor.22178.
Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–56. doi:10.1074/jbc.M302993200.
Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, et al. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181(5):859–71. doi:10.1083/jcb.200708037.
Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280(52):42952–9. doi:10.1074/jbc.M506415200.
Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17(7):1356–67.
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–37.
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117(9):1161–71. doi:10.1161/CIRCULATIONAHA.107.710111.
Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, et al. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17(4):1015–25.
Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, et al. Polycystin-1 and −2 dosage regulates pressure sensing. Cell. 2009;139(3):587–96. doi:10.1016/j.cell.2009.08.045.
Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, et al. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol. 2010;48(1):83–9. doi:10.1016/j.yjmcc.2009.03.020.
Temiyasathit S, Jacobs CR. Osteocyte primary cilium and its role in bone mechanotransduction. Ann N Y Acad Sci. 2010;1192(1):422–8. doi:10.1111/j.1749-6632.2009.05243.x.
Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011;25(7):2418–32.
Leucht P, Monica SD, Temiyasathit S, Lenton K, Manu A, Longaker MT, et al. Primary cilia act as mechanosensors during bone healing around an implant. Med Eng Phys. 2013;35(3):392–402. doi:10.1016/j.medengphy.2012.06.005.
Xiao Z, Zhang S, Cao L, Qiu N, David V, Quarles LD. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J Biol Chem. 2010;285(2):1177–87.
Xiao Z, Zhang S, Magenheimer BS, Luo J, Quarles LD. Polycystin-1 regulates skeletogenesis through stimulation of the osteoblast-specific transcription factor RUNX2-II. J Biol Chem. 2008;283(18):12624–34. doi:10.1074/jbc.M710407200.
Qiu N, Xiao Z, Cao L, David V, Quarles LD. Conditional mesenchymal disruption of pkd1 results in osteopenia and polycystic kidney disease. PLoS One. 2012;7(9):e46038. doi:10.1371/journal.pone.0046038.
Qiu N, Zhou H, Xiao Z. Downregulation of PKD1 by shRNA results in defective osteogenic differentiation via cAMP/PKA pathway in human MG-63 cells. J Cell Biochem. 2012;113(3):967–76. doi:10.1002/jcb.23426.
Arac D, Aust G, Calebiro D, Engel FB, Formstone C, Goffinet A, et al. Dissecting signaling and functions of adhesion G protein-coupled receptors. Ann N Y Acad Sci. 2012;1276:1–25. doi:10.1111/j.1749-6632.2012.06820.x.
Arac D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Sudhof TC, et al. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 2012;31(6):1364–78. doi:10.1038/emboj.2012.26.
Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16(2):179–83.
Qian F, Wei W, Germino G, Oberhauser A. The nanomechanics of polycystin-1 extracellular region. J Biol Chem. 2005;280(49):40723–30.
Oatley P, Stewart AP, Sandford R, Edwardson JM. Atomic force microscopy imaging reveals the domain structure of polycystin-1. Biochemistry. 2012;51(13):2879–88. doi:10.1021/bi300134b.
Dalagiorgou G, Basdra EK, Papavassiliou AG. Polycystin-1: function as a mechanosensor. Int J Biochem Cell Biol. 2010;42(10):1610–3. doi:10.1016/j.biocel.2010.06.017.
Xiao Z, Zhang S, Magenheimer BS, Luo J, Quarles LD. Polycystin-1 regulates skeletogenesis through stimulation of the osteoblast-specific transcription factor Runx2-II. J Biol Chem. 2008;283:12624–34.
Mesner LD, Ray B, Hsu YH, Manichaikul A, Lum E, Bryda EC, et al. Bicc1 is a genetic determinant of osteoblastogenesis and bone mineral density. J Clin Invest. 2014;124(6):2736–49. doi:10.1172/JCI73072.
Qiu N, Cao L, David V, Darryl Quarles L, Xiao Z. Kif3a deficiency reverses the skeletal abnormalities in Pkd1 deficient mice by restoring the balance between osteogenesis and adipogenesis. PLoS ONE. 2010;5(12):e15240.
Qiu N, Xiao Z, Cao L, Buechel MM, David V, Roan E, et al. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 2012;125(8):1945–57.
Qiu N, Zhou H, Xiao Z. Downregulation of PKD1 by shRNA results in defective osteogenic differentiation via cAMP/PKA pathway in human MG-63 cells. J Cell Biochem. 2012;113(3):967–76.
Wann AK, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012;26(4):1663–71. doi:10.1096/fj.11-193649.
Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011;25(7):2418–32. doi:10.1096/fj.10-180299.
Qiu N, Xiao Z, Cao L, Buechel MM, David V, Roan E, et al. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 2012;125(Pt 8):1945–57. doi:10.1242/jcs.095893.
Qiu N, Cao L, David V, Quarles LD, Xiao Z. Kif3a deficiency reverses the skeletal abnormalities in Pkd1 deficient mice by restoring the balance between osteogenesis and adipogenesis. PLoS One. 2010;5(12):e15240. doi:10.1371/journal.pone.0015240.
Xiao Z, Zhang S, Cao L, Qiu N, David V, Quarles LD. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J Biol Chem. 2010;285(2):1177–87. doi:10.1074/jbc.M109.050906.
Xiao ZS, Zhang SQ, Magenheimer BS, Calvet JP, Quarles LD. Polycystin-1 slective activation of Runx2-II isoform transcription is mediated through the calcium-PI3K/Akt pathway. J Bone Miner Res. 2007;22(Suppl 1):S41.
Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13(12):1490–5.
Xiao Z, Cao L, Liang Y, Huang J, Stern AR, Dallas M, et al. Osteoblast-specific deletion of Pkd2 leads to low-turnover osteopenia and reduced bone marrow adiposity. PLoS One. 2014;9(12):e114198. doi:10.1371/journal.pone.0114198.
Dalagiorgou G, Piperi C, Georgopoulou U, Adamopoulos C, Basdra EK, Papavassiliou AG. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell Mol Life Sci. 2013;70(1):167–80. doi:10.1007/s00018-012-1164-5.
Wang H, Sun W, Ma J, Pan Y, Wang L, Zhang W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS One. 2014;9(3):e91730. doi:10.1371/journal.pone.0091730.
Khonsari RH, Ohazama A, Raouf R, Kawasaki M, Kawasaki K, Porntaveetus T, et al. Multiple postnatal craniofacial anomalies are characterized by conditional loss of polycystic kidney disease 2 (Pkd2). Hum Mol Genet. 2013;22(9):1873–85. doi:10.1093/hmg/ddt041.
Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, et al. Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet. 2001;10(21):2385–96.
Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98(21):12174–9.
Turco AE, Padovani EM, Chiaffoni GP, Peissel B, Rossetti S, Marcolongo A, et al. Molecular genetic diagnosis of autosomal dominant polycystic kidney disease in a newborn with bilateral cystic kidneys detected prenatally and multiple skeletal malformations. J Med Genet. 1993;30(5):419–22.
Lindergard B, Johnell O, Nilsson BE, Wiklund PE. Studies of bone morphology, bone densitometry and laboratory data in patients on maintenance hemodialysis treatment. Nephron. 1985;39(2):122–9.
Pavik I, Jaeger P, Kistler AD, Poster D, Krauer F, Cavelti-Weder C, et al. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2011;79(2):234–40. doi:10.1038/ki.2010.375.
Losekoot M, Ruivenkamp CA, Tholens AP, Grimbergen JE, Vijfhuizen L, Vermeer S, et al. Neonatal onset autosomal dominant polycystic kidney disease (ADPKD) in a patient homozygous for a PKD2 missense mutation due to uniparental disomy. J Med Genet. 2012;49(1):37–40. doi:10.1136/jmedgenet-2011-100452.
McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26(4):234–46.
Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, et al. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 2003;227(1):78–90.
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1(4):e53.
Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38(1):112–7.
Tayeh MK, Yen HJ, Beck JS, Searby CC, Westfall TA, Griesbach H, et al. Genetic interaction between Bardet-Biedl syndrome genes and implications for limb patterning. Hum Mol Genet. 2008;17(13):1956–67. doi:10.1093/hmg/ddn093.
Kaushik AP, Martin JA, Zhang Q, Sheffield VC, Morcuende JA. Cartilage abnormalities associated with defects of chondrocytic primary cilia in Bardet-Biedl syndrome mutant mice. J Orthop Res. 2009;27(8):1093–9. doi:10.1002/jor.20855.
Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81(1):170–9. doi:10.1086/519494.
Khaddour R, Smith U, Baala L, Martinovic J, Clavering D, Shaffiq R, et al. Spectrum of MKS1 and MKS3 mutations in Meckel syndrome: a genotype-phenotype correlation. Mutation in brief #960. Online. Hum Mutat. 2007;28(5):523–4. doi:10.1002/humu.9489.
Xiao ZS, Quarles LD. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann N Y Acad Sci. 2010;1192:410–21. doi:10.1111/j.1749-6632.2009.05239.x.
Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–30. doi:10.1073/pnas.0700636104.
Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, et al. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134(11):2159–69. doi:10.1242/dev.001586.
Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24(8):2859–68. doi:10.1096/fj.09-148007.
Uzbekov RE, Maurel DB, Aveline PC, Pallu S, Benhamou CL, Rochefort GY. Centrosome fine ultrastructure of the osteocyte mechanosensitive primary cilium. Microsc Microanal Off J Microsc Soc Am Microbeam Anal Soc Microsc Soc Can. 2012;18(6):1430–41. doi:10.1017/S1431927612013281.
Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104: 13325–13330.
Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, et al. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 2012;7(3):e33368. doi:10.1371/journal.pone.0033368.
Burr DB. The 35th International Sun Valley Workshop on Skeletal Tissue Biology. J Musculoskelet Neuronal Interact. 2005;5(4):307–8.
Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134(2):307–16.
Lee KL, Hoey DA, Spasic M, Tang T, Hammond HK, Jacobs CR. Adenylyl cyclase 6 mediates loading-induced bone adaptation in vivo. FASEB J. 2014;28(3):1157–65. doi:10.1096/fj.13-240432.
O'Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, et al. An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia. 2013;2(1):8. doi:10.1186/2046-2530-2-8.
Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol. 2008;294(3):F542–53. doi:10.1152/ajprenal.00201.2007.
Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, et al. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A. 2007;104(5):1631–6. doi:10.1073/pnas.0605266104.
Cui CB, Cooper LF, Yang X, Karsenty G, Aukhil I. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol Cell Biol. 2003;23(3):1004–13.
Jung H, Lee MS, Jang EJ, Ahn JH, Kang NS, Yoo SE, et al. Augmentation of PPARgamma-TAZ interaction contributes to the anti-adipogenic activity of KR62980. Biochem Pharmacol. 2009;78(10):1323–9. doi:10.1016/j.bcp.2009.07.001.
Yang JY, Cho SW, An JH, Jung JY, Kim SW, Kim SY, et al. Osteoblast-targeted overexpression of TAZ increases bone mass in vivo. PLoS One. 2013;8(2):e56585. doi:10.1371/journal.pone.0056585.
Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309(5737):1074–8. doi:10.1126/science.1110955.
Hao J, Zhang Y, Wang Y, Ye R, Qiu J, Zhao Z, et al. Role of extracellular matrix and YAP/TAZ in cell fate determination. Cell Signal. 2014;26(2):186–91. doi:10.1016/j.cellsig.2013.11.006.
Merrick D, Bertuccio CA, Chapin HC, Lal M, Chauvet V, Caplan MJ. Polycystin-1 cleavage and the regulation of transcriptional pathways. Pediatr Nephrol. 2014;29(4):505–11. doi:10.1007/s00467-013-2548-y.
Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, et al. TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol. 2007;27(18):6383–95. doi:10.1128/MCB.00254-07.
Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114(10):1433–43.
Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. J Cell Biol. 2010;191(4):701–10. doi:10.1083/jcb.201006173.
Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35(1):162–9. doi:10.1016/j.bone.2004.02.018.
Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–711. doi:10.1074/jbc.M601000200.
Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H. Inactivation of Lrp5 in osteocytes reduces young's modulus and responsiveness to the mechanical loading. Bone. 2013;54(1):35–43. doi:10.1016/j.bone.2013.01.033.
Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49(2):184–93. doi:10.1016/j.bone.2011.03.683.
Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O'Brien CA, Manolagas SC, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem. 2007;282(35):25501–8.
Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282(28):20715–27.
Windahl SH, Saxon L, Borjesson AE, Lagerquist MK, Frenkel B, Henning P, et al. Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J Bone Miner Res. 2013;28(2):291–301. doi:10.1002/jbmr.1754.
Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107(31):13648–53. doi:10.1073/pnas.1009382107.
Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600. doi:10.1038/nrm3416.
Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20(7):1103–13. doi:10.1359/JBMR.050210.
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–8. doi:10.1074/jbc.M602308200.
Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25(12):2657–68. doi:10.1002/jbmr.168.
Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, et al. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29(3):705–15. doi:10.1002/jbmr.2064.
Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–62.
Huo B, Lu XL, Costa KD, Xu Q, Guo XE. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium. 2010;47(3):234–41. doi:10.1016/j.ceca.2009.12.005.
Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–14. doi:10.1002/jcp.21021.
Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, et al. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142(8):3464–73. doi:10.1210/endo.142.8.8338.
Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts–correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217(2):640–8.
Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15(2):209–17. doi:10.1359/jbmr.2000.15.2.209.
Ziambaras K, Lecanda F, Steinberg TH, Civitelli R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res. 1998;13(2):218–28. doi:10.1359/jbmr.1998.13.2.218.
Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292(1):C545–52. doi:10.1152/ajpcell.00611.2005.
Batra N, Kar R, Jiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta. 2012;1818(8):1909–18. doi:10.1016/j.bbamem.2011.09.018.
Loiselle AE, Jiang JX, Donahue HJ. Gap junction and hemichannel functions in osteocytes. Bone. 2013;54(2):205–12. doi:10.1016/j.bone.2012.08.132.
Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6(8):e23516. doi:10.1371/journal.pone.0023516.
Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31(7):1075–81. doi:10.1002/jor.22341.
Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27(11):2359–72. doi:10.1002/jbmr.1687.
Grimston SK, Goldberg DB, Watkins M, Brodt MD, Silva MJ, Civitelli R. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res. 2011;26(9):2151–60. doi:10.1002/jbmr.425.
Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57(1):76–83. doi:10.1016/j.bone.2013.07.022.
Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, et al. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22(8):1240–51. doi:10.1091/mbc.E10-07-0571.
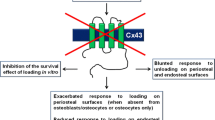
Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27(2):374–89. doi:10.1002/jbmr.548.
Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104(9):1123–30. doi:10.1161/CIRCRESAHA.108.192930.
Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109(9):3359–64. doi:10.1073/pnas.1115967109.
Batra N, Jiang JX. “INTEGRINating” the connexin hemichannel function in bone osteocytes through the action of integrin alpha5. Commun Integr Biol. 2012;5(5):516–8. doi:10.4161/cib.21322.
Klein-Nulend J, Bacabac RG, Bakker AD. Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur Cells Mater. 2012;24:278–91.
Marie PJ. Targeting integrins to promote bone formation and repair. Nat Rev Endocrinol. 2013;9(5):288–95. doi:10.1038/nrendo.2013.4.
Litzenberger JB, Kim JB, Tummala P, Jacobs CR. Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif Tissue Int. 2010;86(4):325–32. doi:10.1007/s00223-010-9343-6.
Wu H, Teng PN, Jayaraman T, Onishi S, Li J, Bannon L, et al. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J Biol Chem. 2011;286(34):29462–9. doi:10.1074/jbc.M110.194746.
McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken). 2009;292(3):355–63. doi:10.1002/ar.20869.
Watabe H, Furuhama T, Tani-Ishii N, Mikuni-Takagaki Y. Mechanotransduction activates alpha(5)beta(1) integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell Res. 2011;317(18):2642–9. doi:10.1016/j.yexcr.2011.07.015.
Carvalho RS, Bumann A, Schaffer JL, Gerstenfeld LC. Predominant integrin ligands expressed by osteoblasts show preferential regulation in response to both cell adhesion and mechanical perturbation. J Cell Biochem. 2002;84(3):497–508.
Young SR, Gerard-O'Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24(3):411–24. doi:10.1359/jbmr.081102.
Hamidouche Z, Fromigue O, Ringe J, Haupl T, Vaudin P, Pages JC, et al. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009;106(44):18587–91. doi:10.1073/pnas.0812334106.
Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol. 2000;220(1):2–15. doi:10.1006/dbio.2000.9633.
Iwaniec UT, Wronski TJ, Amblard D, Nishimura Y, van der Meulen MC, Wade CE, et al. Effects of disrupted beta1-integrin function on the skeletal response to short-term hindlimb unloading in mice. J Appl Physiol. 2005;98(2):690–6. doi:10.1152/japplphysiol.00689.2004.
Phillips JA, Almeida EA, Hill EL, Aguirre JI, Rivera MF, Nachbandi I, et al. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 2008;27(7):609–18. doi:10.1016/j.matbio.2008.05.003.
Shekaran A, Shoemaker JT, Kavanaugh TE, Lin AS, LaPlaca MC, Fan Y, et al. The effect of conditional inactivation of beta 1 integrins using twist 2 Cre, Osterix Cre and osteocalcin Cre lines on skeletal phenotype. Bone. 2014;68:131–41. doi:10.1016/j.bone.2014.08.008.
Srouji S, Ben-David D, Fromigue O, Vaudin P, Kuhn G, Muller R, et al. Lentiviral-mediated integrin alpha5 expression in human adult mesenchymal stromal cells promotes bone repair in mouse cranial and long-bone defects. Hum Gene Ther. 2012;23(2):167–72. doi:10.1089/hum.2011.059.
Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48(4):330–8. doi:10.1016/j.ultras.2008.02.005.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biolumin Chemilumin. 2008;283(9):5866–75. doi:10.1074/jbc.M705092200.
Rath AL, Bonewald LF, Ling J, Jiang JX, Van Dyke ME, Nicolella DP. Correlation of cell strain in single osteocytes with intracellular calcium, but not intracellular nitric oxide, in response to fluid flow. J Biomech. 2010. doi:10.1016/j.jbiomech.2010.01.030.
Lee DS, Choung HW, Kim HJ, Gronostajski RM, Yang YI, Ryoo HM, et al. NFI-C regulates osteoblast differentiation via control of osterix expression. Stem Cells. 2014;32(9):2467–79. doi:10.1002/stem.1733.
Waki H, Nakamura M, Yamauchi T, Wakabayashi K, Yu J, Hirose-Yotsuya L, et al. Global mapping of cell type-specific open chromatin by FAIRE-seq reveals the regulatory role of the NFI family in adipocyte differentiation. PLoS Genet. 2011;7(10):e1002311. doi:10.1371/journal.pgen.1002311.
Niziolek PJ, Farmer TL, Cui Y, Turner CH, Warman ML, Robling AG. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone. 2011;49(5):1010–9. doi:10.1016/j.bone.2011.07.034.
Boca M, D'Amato L, Distefano G, Polishchuk RS, Germino GG, Boletta A. Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Mol Biol Cell. 2007;18(10):4050–61. doi:10.1091/mbc.E07-02-0142.
Boca M, Distefano G, Qian F, Bhunia AK, Germino GG, Boletta A. Polycystin-1 induces resistance to apoptosis through the phosphatidylinositol 3-kinase/Akt signaling pathway. J Am Soc Nephrol. 2006;17(3):637–47. doi:10.1681/ASN.2005050534.
Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, et al. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274(8):4947–53.
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating RUNX2 gene expression. J Biol Chem. 2005;280:33132–40.
Chang CF, Serra R. Ift88 regulates Hedgehog signaling, Sfrp5 expression, and beta-catenin activity in post-natal growth plate. J Orthop Res. 2013;31(3):350–6. doi:10.1002/jor.22237.
May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia. 2012;1(1):7. doi:10.1186/2046-2530-1-7.
Gerdes JM, Katsanis N. Ciliary function and Wnt signal modulation. Curr Top Dev Biol. 2008;85:175–95. doi:10.1016/S0070-2153(08)00807-7.
Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10(1):70–6. doi:10.1038/ncb1670.
Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol. 2011;13(6):700–7. doi:10.1038/ncb2259.
Astudillo P, Larrain J. Wnt signaling and cell-matrix adhesion. Curr Mol Med. 2014;14(2):209–20.
Amin N, Vincan E. The Wnt signaling pathways and cell adhesion. Front Biosci. 2012;17:784–804.
Astudillo P, Carrasco H, Larrain J. Syndecan-4 inhibits Wnt/beta-catenin signaling through regulation of low-density-lipoprotein receptor-related protein (LRP6) and R-spondin 3. Int J Biochem Cell Biol. 2014;46:103–12. doi:10.1016/j.biocel.2013.11.012.
Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–56. doi:10.1016/j.cell.2012.11.027.
Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB, et al. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014;21(6):854–63. doi:10.1038/cdd.2014.8.
Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A, Ikeda K. Integrin alphav in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447(2):352–7. doi:10.1016/j.bbrc.2014.04.006.
Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes Dev. 2015;29(1):1–6. doi:10.1101/gad.253682.114.
Reich KM, McAllister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology. 1997;138(3):1014–8.
Garcia M, Knight MM. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res. 2010;28(4):510–5. doi:10.1002/jor.21025.
Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, et al. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res. 1999;14(7):1123–31. doi:10.1359/jbmr.1999.14.7.1123.
Mehrotra M, Saegusa M, Voznesensky O, Pilbeam C. Role of Cbfa1/Runx2 in the fluid shear stress induction of COX-2 in osteoblasts. Biochem Biophys Res Commun. 2006;341(4):1225–30. doi:10.1016/j.bbrc.2006.01.084.
Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11(11):1688–93.
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, et al. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104(7):860–9. doi:10.1161/CIRCRESAHA.108.192765.
Xu H, Guan Y, Wu J, Zhang J, Duan J, An L, et al. Polycystin 2 is involved in the nitric oxide production in responding to oscillating fluid shear in MLO-Y4 cells. J Biomech. 2014;47(2):387–91. doi:10.1016/j.jbiomech.2013.11.018.
Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 2014;28(1):430–9. doi:10.1096/fj.13-231894.
Hoey DA, Tormey S, Ramcharan S, O'Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30(11):2561–70. doi:10.1002/stem.1235.
Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104(11):4389–94. doi:10.1073/pnas.0700499104.
Turner CH, Robling AG. Exercise as an anabolic stimulus for bone. Curr Pharm Des. 2004;10(21):2629–41.
Liu PY, Brummel-Smith K, Ilich JZ. Aerobic exercise and whole-body vibration in offsetting bone loss in older adults. J Aging Res. 2011;2011:379674. doi:10.4061/2011/379674.
Smith SM, Zwart SR, Heer M, Hudson EK, Shackelford L, Morgan JL. Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J Bone Miner Res. 2014. doi:10.1002/jbmr.2185.
Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27(9):1896–906. doi:10.1002/jbmr.1647.
Martinez de Albornoz P, Khanna A, Longo UG, Forriol F, Maffulli N. The evidence of low-intensity pulsed ultrasound for in vitro, animal and human fracture healing. Br Med Bull. 2011;100:39–57. doi:10.1093/bmb/ldr006.
Griffin XL, Smith N, Parsons N, Costa ML. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst Rev. 2012;2:CD008579. doi:10.1002/14651858.CD008579.pub2.
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–4. doi:10.1038/35088122.
Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15(12):2225–9. doi:10.1096/fj.01-0166com.
Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17(2):349–57. doi:10.1359/jbmr.2002.17.2.349.
Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21(9):1464–74. doi:10.1359/jbmr.060612.
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–51. doi:10.1359/JBMR.0301251.
Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37(4):411–7.
Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 4. Mechanical influences on intact fibrous tissues. Anat Rec. 1990;226(4):433–9. doi:10.1002/ar.1092260405.
Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30(5):693–702.
Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30(3):445–52.
Acknowledgments
Supported by grant from NIH (DK083303).
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, Z., Quarles, L.D. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev Endocr Metab Disord 16, 115–129 (2015). https://doi.org/10.1007/s11154-015-9313-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-015-9313-4