Abstract
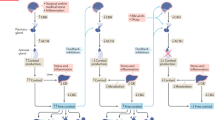
Hypothalamic-pituitary-adrenal (HPA) axis function is crucial to maintain and restore homeostasis. The HPA axis does not function in isolation. Rather, the HPA axis modulates and reacts to signals from endocrine, neural, and immune systems. Cortisol is the major glucocorticoid secreted by the human adrenal cortex. Its actions are largely mediated by the glucocorticoid receptor. The potent anti-inflammatory actions of glucocorticoids led to their use in critically ill patients. Metaanalyses of these early studies (before 1985) concluded that large glucocorticoid doses had no effect and were potentially detrimental. More recently, the pendulum has swung in the opposite direction based on the concept that critically ill patients may have relative adrenal insufficiency and/or acquired glucocorticoid resistance. However, inconsistent diagnostic criteria, heterogeneity of subjects, variable nutritional status, and pre-existing conditions preclude formulating definitive conclusions regarding glucocorticoid use among critically patients. Diagnosing adrenal insufficiency in the critically ill patient remains challenging. To resolve the issue, our challenge is to develop physiologically relevant tools to assess glucocorticoid action and GR function at the cellular level.
Similar content being viewed by others
References
Selye H.. A syndrome produced by diverse noxious agents. London: Nature; 1936. p. 138–2.
Turrin NP, Rivest S. Unraveling the molecular details involved in the intimate link between the immune and neuroendocrine systems. Exp Biol Med (Maywood). 2004;229:996–1006.
Chrousos GP. The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62.
Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Reviews in Endocrine and Metabolic Disorders 2007; DOI 10.1007/s11154-007-9052-2.
Hammond GL, Smith CL, Paterson NA, Sibbald WJ. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990;71:34–9.
Brien TG. Human corticosteroid binding globulin. Clin Endocrinol (Oxf). 1981;14:193–212.
Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, et al. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–66.
Tomlinson JW, Moore J, Cooper MS, Bujalska I, Shahmanesh M, Burt C, et al. Regulation of expression of 11ß-hydroxysteroid dehydrogenase type 1 in adipose tissue: tissue-specific induction by cytokines. Endocrinology. 2001;142:1982–9.
Seckl JR. 11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action. Curr Opin Pharmacol. 2004;4:597–602.
Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC, et al. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res. 2001;16:1037–44.
Palermo M, Gomez-Sanchez C, Roitman E, Shackleton CH. Quantitation of cortisol and related 3-oxo-4-ene steroids in urine using gas chromatography/mass spectrometry with stable isotope-labeled internal standards. Steroids. 1996;61:583–9.
Kornel L, Miyabo S. Studies on steroid conjugates: IX. Urinary excretion of sulfate conjugated metabolites of cortisol in man. Steroids. 1975;25:697–706.
DeFranco DB. Navigating steroid hormone receptors through the nuclear compartment. Mol Endocrinol. 2002;16:1449–55.
McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59.
De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522.
Reichardt HM, Kaestner KH, Wessely O, Gass P, Schmid W, Schutz G. Analysis of glucocorticoid signalling by gene targeting. J Steroid Biochem Mol Biol. 1998;65:111–15.
Stellato C. Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc. 2004;1:255–63.
Limbourg FP, Liao JK. Nontranscriptional actions of the glucocorticoid receptor. J Mol Med. 2003;81:168–74.
Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol. 2003;284:R628–38.
Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol. 2006;102:11–21.
Pujols L, Mullol J, Picado C. Alpha and beta glucocorticoid receptors: relevance in airway diseases. Curr Allergy Asthma Rep. 2007;7:93–9.
Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16:301–7.
Jewell CM, Cidlowski JA. Molecular evidence for a link between the N363S glucocorticoid receptor polymorphism and altered gene expression. J Clin Endocrinol Metab. 2007;92:3268–77.
Croxtall JD, van Hal PT, Choudhury Q, Gilroy DW, Flower RJ. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br J Pharmacol. 2002;135:511–9.
Päth G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab. 1997;82:2343–9.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23.
Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303 (Jun).
Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–37.
Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813.
Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9.
Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol. 2007;21:1267–80.
Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol. 2005;35:3405–13.
Berdeli A, Mir S, Ozkayin N, Serdaroglu E, Tabel Y, Cura A. Association of macrophage migration inhibitory factor-173C allele polymorphism with steroid resistance in children with nephrotic syndrome. Pediatr Nephrol. 2005;20:1566–71.
Griga T, Wilkens C, Wirkus N, Epplen J, Schmiegel W, Klein W. A polymorphism in the macrophage migration inhibitory factor gene is involved in the genetic predisposition of Crohn's disease and associated with cumulative steroid doses. Hepatogastroenterology. 2007;54:784–6.
Martich GD, Boujoukos AJ, Suffredini AF. Response of man to endotoxin. Immunobiology. 1993;187:403–16.
Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89.
van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3604–6.
Udelsman R, Norton JA, Jelenich SE, Goldstein DS, Linehan WM, Loriaux DL, Chrousos GP. Responses of the hypothalamic–pituitary–adrenal and renin–angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64:986–94.
Naito Y, Tamai S, Shingu K, Shindo K, Matsui T, Segawa H, Nakai Y, Mori K. Responses of plasma adrenocorticotropic hormone, cortisol, and cytokines during and after upper abdominal surgery. Anesthesiology. 1992;77:426–31.
Naito Y, Fukata J, Tamai S, Seo N, Nakai Y, Mori K, et al. Biphasic changes in hypothalamo–pituitary–adrenal function during the early recovery period after major abdominal surgery. J Clin Endocrinol Metab. 1991;73:111–7.
Vermes I, Beishuizen A, Hampsink RM, Haanen C. Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab. 1995;80:1238–42.
Beishuizen A, Thijs LG, Haanen C, Vermes I. Macrophage migration inhibitory factor and hypothalamo–pituitary–adrenal function during critical illness. J Clin Endocrinol Metab. 2001;86:2811–6.
Sprong T, Pickkers P, Geurts-Moespot A, van der Ven-Jongekrijg J, Neeleman C, Knaup M, et al. Macrophage migration inhibitory factor (MIF) in meningococcal septic shock and experimental human endotoxemia. Shock. 2007;27:482–7.
Tsigos C, Kyrou I, Chrousos GP, Papanicolaou DA. Prolonged suppression of corticosteroid-binding globulin by recombinant human interleukin-6 in man. J Clin Endocrinol Metab. 1998;83:3379.
Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–21.
Reichardt HM, Umland T, Bauer A, Kretz O, Schutz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–17.
Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood. 2007;109:4313–9.
Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–6.
Vakharia K, Hinson JP. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology. 2005;146:1398–402.
Bornstein SR, Schumann RR, Rettori V, McCann SM, Zacharowski K. Toll-like receptor 2 and Toll-like receptor 4 expression in human adrenals. Horm Metab Res. 2004;36:470–3.
Tran N, Koch A, Berkels R, Boehm O, Zacharowski PA, Baumgarten G, et al. Toll-like receptor 9 expression in murine and human adrenal glands and possible implications during inflammation. J Clin Endocrinol Metab. 2007;92:2773–83.
Bornstein SR, Zacharowski P, Schumann RR, Barthel A, Tran N, Papewalis C, et al. Impaired adrenal stress response in Toll-like receptor 2-deficient mice. Proc Natl Acad Sci U S A. 2004;101:16695–700.
Zacharowski K, Zacharowski PA, Koch A, Baban A, Tran N, Berkels R, et al. Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci U S A. 2006;103:6392–7.
Carpenter S, O, neill LA. How important are toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901.
O’Neill LA. How toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9.
Burgner D, Jamieson SE, Blackwell JM. Genetic susceptibility to infectious diseases: big is beautiful, but will bigger be even better? Lancet Infect Dis. 2006;6:653–63.
Mockenhaupt FP, Cramer JP, Hamann L, Stegemann MS, Eckert J, Oh NR, et al. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci U S A. 2006;103:177–82.
Smirnova I, Mann N, Dols A, Derkx HH, Hibberd ML, Levin M, et al. Assay of locus-specific genetic load implicates rare toll-like receptor 4 mutations in meningococcal susceptibility. Proc Natl Acad Sci U S A. 2003;100:6075–80.
Charmandari E, Kino T, Souvatzoglou E, Vottero A, Bhattacharyya N, Chrousos GP. Natural glucocorticoid receptor mutants causing generalized glucocorticoid resistance: molecular genotype, genetic transmission, and clinical phenotype. J Clin Endocrinol Metab. 2004;89:1939–49.
Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–21.
den Brinker M, Hokken-Koelega AC, Hazelzet JA, de Jong FH, Hop WC, Joosten KF. One single dose of etomidate negatively influences adrenocortical performance for at least 24 hours in children with meningococcal sepsis. Intensive Care Med. 2007 Aug 21; DOI 10.1007/s00134-007-0836-3
Balk RA. Steroids for septic shock: back from the dead? (Pro) Chest. 2003;123(5 Suppl):490S–9S.
Waterhouse R. A case of suprarenal apoplexy. Lancet. 1911;1:577–8.
Nelson J, Goldstein N. Nature of Waterhouse-Friderichsen syndrome: report of case with successful treatment with cortisone. JAMA. 1951;146:1193–7.
Shane SJ, Gaum AR, Gaum D. Acute adrenal insufficiency (Waterhouse-Friderichsen syndrome) treated with cortisone. Can Med Assoc J. 1955;72:372–3.
Weitzman S, Berger S. Clinical trial design in studies of corticosteroids for bacterial infections. Ann Intern Med. 1974;81:36–42.
Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333–41.
Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med. 1984;311:1137–43.
Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–8.
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–8.
The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659–65.
Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MAD, et al. Corticosteroid treatment of sepsis: a critical appraisal and metaanalysis of the literature. Crit Care Med. 1995;23:1430–9.
Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–303.
Moran JL, Chapman MJ, O, Fathartaigh MS, Peisach AR, Pannall PR, Leppard P. Hypocortisolaemia and adrenocortical responsiveness at onset of septic shock. Intensive Care Med. 1994;20:489–95.
Bouachour G, Tirot P, Gouello JP, Mathieu E, Vincent JF, Alquier P. Adrenocortical function during septic shock. Intensive Care Med. 1995;21:57–62.
Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31:141–5.
Aygen B, Inan M, Doganay M, Kelestimur F. Adrenal functions in patients with sepsis. Exp Clin Endocrinol Diabetes. 1997;105:182–6.
Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet. 1991;337:582–3.
Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–45.
Rady MY, Johnson DJ, Patel B, Larson J, Helmers R. Cortisol levels and corticosteroid administration fail to predict mortality in critical illness: the confounding effects of organ dysfunction and sex. Arch Surg. 2005;140:661–8.
Sam S, Corbridge TC, Mokhlesi B, Comellas AP, Molitch ME. Cortisol levels and mortality in severe sepsis. Clin Endocrinol (Oxf). 2004;60:29–35.
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71.
Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–45.
Annane D, Bellissant E, Sebille V, Lesieur O, Mathieu B, Raphael JC, et al. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. Br J Clin Pharmacol. 1998;46:589–97.
Schneider AJ, Voerman HJ. Abrupt hemodynamic improvement in late septic shock with physiological doses of glucocorticoids. Intensive Care Med. 1991;17:436–7.
Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, et al. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clin Investig. 1994;72:782–7.
Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723–32.
Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–50.
Oppert M, Schindler R, Husung C, Offermann K, Graf KJ, Boenisch O, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33:2457–64.
Burry LD, Wax RS. Role of corticosteroids in septic shock. Ann Pharmacother. 2004;38:464–72.
Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141:47–56.
Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: The retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–18.
Britt RC, Devine A, Swallen KC, Weireter LJ, Collins JN, Cole FJ, et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141:145–49.
Gannon TA, Britt RC, Weireter LJ, Cole FJ, Collins JN, Britt LD. Adrenal insufficiency in the critically ill trauma population. Am Surg. 2006;72:373–6.
DeMaria EJ, Reichman W, Kenney PR, Armitage JM, Gann DS. Septic complications of corticosteroid administration after central nervous system trauma. Ann Surg. 1985;202:248–52.
Pizarro, Troster, Damiani, Carcilli. Absolute and relative adrenal insufficiency with septic shock. Critical Care Med. 2005;33:911–12.
Markovitz BP, Goodman DM, Watson RS, Bertoch D, Zimmerman J. A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: what is the role of steroids? Pediatric Critical Care Med. 2005;6:270–4.
Sarthi M, Lodha R, Vivekanandhan S, et al. Adrenal status in children with septic shock using low-dose stimulation test. Pediatr Crit Care Med. 2007;8:23–8.
Hatherill M, Tibby SM, Hilliard T, Turner C, Murdoch IA. Adrenal insufficiency in septic shock. Arch Dis Child. 1999;80:51–5.
Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–38.
Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26:197–202.
Ho JT, Al-Musalhi H, Chapman MJ, Quach T, Thomas PD, Bagley CJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91:105–14.
Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353:2352–60.
Tait AS, Dalton M, Geny B, D, Agnillo F, Popoff MR, Sternberg EM. The large clostridial toxins from Clostridium sordellii and C. difficile repress glucocorticoid receptor activity. Infect Immun. 2007;75:3935–40.
Webster JI, Sternberg EM. Anthrax lethal toxin represses glucocorticoid receptor (GR) transactivation by inhibiting GR-DNA binding in vivo. Mol Cell Endocrinol. 2005;241:21–31.
Sessler CN. Steroids for septic shock: back from the dead? (Con) Chest. 2003;123(5 Suppl):482S–9S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkataraman, S., Munoz, R., Candido, C. et al. The hypothalamic–pituitary–adrenal axis in critical illness. Rev Endocr Metab Disord 8, 365–373 (2007). https://doi.org/10.1007/s11154-007-9058-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-007-9058-9