Abstract
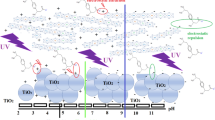
This paper reports on the photodegradation of ibuprofen (IBP), as a model pharmaceutical, using SiO2@xTiO2 synthesized via a hydrothermal method. The catalysts were prepared by adding TiO2 at different weight loadings (2, 5, 10 and 20 wt%) to a Stober synthesized silica (ca. 120 ± 10 nm diameter). The core–shell materials were characterized by TEM, XRD, reflectance diffuse spectroscopy, Raman spectroscopy, BET analysis and electrical conductivity. The photodegradation was performed using UV irradiation (254 nm, 25 W). The effect of catalyst dosage and the pH of the solution on the rate of IBP photodegradation was investigated. The SiO2@xTiO2 showed excellent photodegradation efficiency and the decomposition reaction was found to be pseudo first order where the degradation rate of IBP increased with the formation of H2O2. Optimal experimental conditions gave 99% IBP degradation with the SiO2@20TiO2 catalyst (0.1 g/L, at 180 min of irradiation).








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
References
Gracia-Lor E, Sancho JV, Hernández F (2011) Multi-class determination of around 50 pharmaceuticals, including 26 antibiotics, in environmental and wastewater samples by ultrahigh performance liquid chromatography–tandem mass spectrometry. J Chrom A 1218:2264–2275. https://doi.org/10.1016/j.chroma.2011.02.026
Matongo S, Birungi G, Moodley B, Ndungua P (2015) Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere 134:133–140. https://doi.org/10.1016/j.chemosphere.2015.03.093
Caban M, Lis E, Kumirska J, Stepnowski P (2015) Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS(SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci Total Environ 538:402–411. https://doi.org/10.1016/j.scitotenv.2015.08.076
Sim WJ, Kim HY, Choi SD, Kwon JH, Oh JE (2013) Evaluation of pharmaceuticals and personal care products with emphasis on anthelmintics in human sanitary waste, sewage, hospital wastewater, livestock wastewater and receiving water. J Hazard Mater 248–249:219–227. https://doi.org/10.1016/j.jhazmat.2013.01.007
Pulgarin C, Kiwi J (1996) Overview on photocatalytic and electrocatalytic pretreatment of industrial non-biodegradable pollutants and pesticides. Chimia 50(3):50–55. https://doi.org/10.2533/chimia.1996.50
Ribordy P, Pulgarin C, Kiwi J, Peringer P (1997) Electrochemical versus photochemical pretreatment of industrial wastewaters. Water Sci Technol 35(4):293–302. https://doi.org/10.2166/wst.1997.0141
Herrera F, Pulgarin C, Nadtochenko V, Kiwi J (1998) Accelerated photo-oxidation of concentrated p-coumaric acid in homogeneous solution. Mechanistic studies, intermediates and precursors formed in the dark. Appl Catal B: Environ 17(1–2):141–156. https://doi.org/10.1016/S0926-3373(98)00008-3
Parsons SA, Byrne A (2004) Advanced oxidation processes for water and wastewater treatment. In: Parson S (ed) Water treatment applications. IWA Publishing, London, pp 329–346. https://doi.org/10.2166/9781780403076
Madhavan J, Grieser F, Ashokkumar M (2010) Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J Hazard Mater 178(1–3):202–208. https://doi.org/10.1016/j.jhazmat.2010.01.064
Musa KAK, Eriksson LA (2007) Theoretical study of ibuprofen phototoxicity. J Phys Chem B 111:13345–13352. https://doi.org/10.1021/jp076553e
Skoumal M, Rodriguez RM, Cabot PL, Centellas F, Garrido JA, Arias C, Brillas E (2009) Electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton degradation of the drug ibuprofen in acid aqueous medium using platinum and boron-doped diamond anodes. Electrochim Acta 54:2077–2085. https://doi.org/10.1016/j.electacta.2008.07.014
Madhavan J, Grieser F, Ashokkumar M (2010) Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J Hazard Mater 178:202–208. https://doi.org/10.1016/j.jhazmat.2010.01.064
Buser HR, Poiger T, Muller MD (1999) Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ Sci Technol 33:2529–2535. https://doi.org/10.1021/es981014w
Richardson ML, Bowron JM (1985) The fate of pharmaceutical chemicals in the aquatic environment. J Pharm Pharmacol 37:1–12. https://doi.org/10.1111/j.2042-7158.1985.tb04922.x
Idaka E, Ogawa T, Horitsu H (1987) Reductive metabolism of aminoazobenzenes by Pseudomonas cepacia. Bull Environ Contam Toxicol 39:100–107. https://doi.org/10.1007/BF01691796
Sweeney EA, Chipman JK, Forsythe S (1994) Evidence for direct-acting oxidative genotoxicity by reduction products of azo dyes. J Environ Health Perspect 102:119–122. https://doi.org/10.1289/ehp.94102s6119
Wong PK, Yuen PY (1996) Decolorization and biodegradation of methyl red by Kleb-siellapneumoniae RS-13. Water Res 30:1736–1744. https://doi.org/10.1016/0043-1354(96)00067-X
Esplugas S, Bila DM, Krause LGT, Dezotti M (2007) Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazard Mater 149:631–642. https://doi.org/10.1016/j.jhazmat.2007.07.073
Selli E, Bianchi CL, Pirola C, Cappelletti G, Ragaini V (2008) Efficiency of 1,4- dichloro benzene degradation in water under photolysis, photocatalysis on TiO2 and sonolysis. J Hazard Mater 15:1136–1141. https://doi.org/10.1016/j.jhazmat.2007.09.071
Hoffmann MR, Martin ST, Choi W, Bahnemamm DW (1995) Environmental applications of semiconductor photocatalytic. Chem Rev 95:69–96. https://doi.org/10.1021/cr00033a004
Fujishima A, Zhang X (2006) Titanium dioxide photocatysis: present situation and future approaches. C R Chim 9:750–760. https://doi.org/10.1016/j.crci.2005.02.055
Fujishima A, Zhang X, Tryk DA (2007) Heterogeneous photocatalysis: from water photolysis: from water photolysis to application in environmental cleanup. Int J Hydrogen Energ 32:2664–2672. https://doi.org/10.1016/j.ijhydene.2006.09.009
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1977) Light-induced amphiphic surfaces. Nature 388:431–432. https://doi.org/10.1038/41233
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol C Photochem Rev 13:169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
Łuczak J, Paszkiewicz-Gawron M, Długokęcka M, Lisowski W, Grabowska E, Makurat S, Rak J, Zaleska-Medynska A (2017) Visible-light photocatalytic activity of ionic liquid TiO2 spheres: effect of the ionic liquid’s anion structure. Chem Cat Chem 9:4377–4388. https://doi.org/10.1002/cctc.201700861
Wang D, Hou P, Zhang L, Yang P, Cheng X (2017) Photocatalytic and hydrophobic activity of cement-based materials from benzyl-terminated-TiO2 spheres with core-shell structures. Constr Build Mater 148:176–183. https://doi.org/10.1016/j.conbuildmat.2017.05.038
Dhawale DS, Gujar TP, Lokhande CD (2017) TiO2 nanorods decorated with Pd nanoparticles for enhanced liquefied petroleum gas sensing performance. Anal Chem 89:8531–8537. https://doi.org/10.1021/acs.analchem.7b02312
Dhandole LK, Mahadik MA, Kim SG, Chung HS, Seo YS, Cho M, Ryu JH, Jang JS (2017) Boosting photocatalytic performance of inactive rutile TiO2 nanorods under solar light irradiation: synergistic effect of acid treatment and metal oxide co-catalysts. ACS Appl Mater Interfaces 9:23602–23613. https://doi.org/10.1021/acsami.7b02104
Nie S, Liu D, Liu Y, Yang P (2017) Phase controlling of TiO2 fibers by adjusting precursor pre-hydrolysis and calcined process towards enhanced photocatalysis. J Nanosci Nanotechnol 17:3430–3434. https://doi.org/10.1166/jnn.2017.12808
Hatat-Fraile M, Liang R, Arlos MJ, He RX, Peng P, Servos MR, Zhou YN (2017) Concurrent photocatalytic and filtration processes using doped TiO2 coated quartz fiber membranes in a photocatalytic membrane reactor. Chem Eng J 330:531–540. https://doi.org/10.1016/j.cej.2017.07.141
Mohajernia S, Hejazi S, Mazare A, Nguyen NT, Schmuki P (2017) Photoelectrochemical H2 generation from suboxide TiO2 nanotubes: visible-light absorption versus conductivity. Chem A Eur J 23:12406–12411. https://doi.org/10.1002/chem.201702245
Deyab MA, Nada AA, Hamdy A (2017) Comparative study on the corrosion and mechanical properties of nano-composite coatings incorporated with TiO2 nano-particles, TiO2 nano-tubes, and ZnO nano-flowers. Prog Org Coat 105:245–251. https://doi.org/10.1016/j.porgcoat.2016.12.026
Si J, Wang Y, Xia X, Peng S, Wang Y, Xiao S, Zhu L, Bao Y, Huang Z, Gao Y (2017) Novel quantum dot and nano-sheet TiO2 (B) composite for enhanced photocatalytic H2 – Production without Co-Catalyst. J Power Sources 360:353–359. https://doi.org/10.1016/j.jpowsour.2017.06.021
Kimab CK, Leea GJ, Leea MK, Rhee CK (2014) A novel method to prepare Cu@Ag core–shell nanoparticles for printed flexible electronics. Powder Technol 263:1–6. https://doi.org/10.1016/j.powtec.2014.04.064
Chatterjee K, Sarkar S, Rao KJ, Paria S (2014) Core/shell nanoparticles in biomedical applications. Adv Colloid Interface Sci 209:8–39. https://doi.org/10.1016/j.cis.2013.12.008
Filippousi M, Papadimitriou SA, Bikiaris DN, Pavlidou E, Angelakeris M, Zamboulis D, Tian H, Tendeloo GV (2013) Novel core–shell magnetic nanoparticles for Taxol encapsulation in biodegradable and biocompatible block copolymers: preparation, characterization and release properties. Int J Pharm 448:221–230. https://doi.org/10.1016/j.ijpharm.2013.03.025
Zhaolong W, Xiaojun Q, Zhuomin Z, Ping C (2018) Optical absorption of carbon-gold core-shell nanoparticles. J Quant Spectrosc Radiat Transfer 205:291–298. https://doi.org/10.1016/j.jqsrt.2017.08.001
Song L, Wang Y, Ma J, Zhang Q, Shen Z (2018) Core/shell structured Zn/ZnO nanoparticles synthesized by gaseous laser ablation with enhanced photocatalysis efficiency. Appl Surf Sci 442:101–105. https://doi.org/10.1016/j.apsusc.2018.02.143
Chaudhuri RG, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112:2373–2433. https://doi.org/10.1021/cr100449n
Sreeja S, Shetty KV (2017) Photocatalytic water disinfection under solar irradiation by Ag@TiO2 core-shell structured nanoparticles. Sol Energy 157:236–243. https://doi.org/10.1016/j.solener.2017.07.057
Ma L, Wang G, Jiang C, Bao H, Xu Q (2018) Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl Surf Sci 430:263–272. https://doi.org/10.1016/j.apsusc.2017.07.282
Ferreira-Neto EP, Ullah S, Simões MB, Perissinotto AP, de Vicente FS, Noeske PL, Ribeiro SJ, Rodrigues-Filho UP (2019) Solvent-controlled deposition of titania on silica spheres for the preparation of SiO2@TiO2 core@shell nanoparticles with enhanced photocatalytic activity. Colloids Surf A Physicochem Eng Asp 570:293–305. https://doi.org/10.1016/j.colsurfa.2019.03.036
Yue X, Li H, Qiu Y, Xiao Z, Yu X, Xue C, Xiang J (2021) A facile synthesis method of TiO2@SiO2 porous core shell structure for photocatalytic hydrogen evolution. J Solid State Chem 300:122250. https://doi.org/10.1016/j.jssc.2021.122250
Zhang Y, Li P (2015) Porous Zr-doped SiO 2 shell/TiO 2 core nanoparticles with expanded channels for photocatalysis. Mater Des 88:1250–1259. https://doi.org/10.1016/j.matdes.2015.09.093
Ren Y, Li W, Cao Z, Jiao Y, Xu J, Liu P, Li S, Li., X., (2020) Robust TiO2 nanorods-SiO2 core-shell coating with high-performance self-cleaning properties under visible light. Appl Surf Sci 509:145377. https://doi.org/10.1016/j.apsusc.2020.145377
Fernandes IL, Barbosab DP, de Oliveira SB, da Silva VA, Sousa MH, Munoz MM, Coaquira JAH (2022) Synthesis and characterization of the MNP@SiO2@TiO2 nanocomposite showing strong photocatalytic activity against methylene blue dye. Appl Surf Sci 580:152195. https://doi.org/10.1016/j.apsusc.2021.152195
Khodadadi M, Ehrampoush MH, Ghaneian MT, Allahresani A, Mahvi AH (2018) Synthesis and characterizations of FeNi3 @SiO2 @TiO2 nanocomposite and its application in photo- catalytic degradation of tetracycline in simulated wastewater. J Mol Liq 255:224–232. https://doi.org/10.1016/j.molliq.2017.11.137
Langhuan H, Houjin W, Yingliang L, Caixuan C (2011) Preparation, characterization and photocatalytic activity of Pt-SiO2/TiO2 with core-shell structure. Rare Metal Mater Eng 40(11):1901–1905. https://doi.org/10.1016/S1875-5372(12)60011-3
Jiang Q, Huang J, Ma B, Yang Z, Zhang T, Wang X (2020) Recyclable, hierarchical hollow photocatalyst TiO2@SiO2 composite microsphere realized by raspberry-like SiO2. Colloids Surf A Physicochem Eng Asp 602:125112. https://doi.org/10.1016/j.colsurfa.2020.125112
Ma J, Guo X, Ge H, Tian G, Zhang Q (2018) Seed-mediated photodeposition route to Ag-decorated SiO2 @TiO2 microspheres with ideal core-shell structure and enhanced photocatalytic activity. Appl Surf Sci 434:1007–1014. https://doi.org/10.1016/j.apsusc.2017.11.020
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interf Sci 26:62–69. https://doi.org/10.1016/0021-9797(68)90272-5
Kerchich S, Boudjemaa A, Chebout R, Bachari K, Mameri N (2021) High performance of δ-Fe2O3 novel photo-catalyst supported on LDH structure. J Photochem Photobiol A Chem 406:113001. https://doi.org/10.1016/j.jphotochem.2020.113001
Cheng J, Wang Y, Xing Y, Shahid M, Pan W (2017) A stable and highly efficient visible-light photocatalyst of TiO2 and heterogeneous carboncore–shell nano fibers. Nanomaterials 7:289–293. https://doi.org/10.1039/C7RA00546F
Boudjemaa A, Nongwe I, Mutuma BK, Matsoso BJ, Bachari K, Coville NJ (2021) TiO2@hollow carbon spheres: a photocatalyst for hydrogen generation under visible irradiation. J Photochem Photobiol A Chem 417:113355. https://doi.org/10.1016/j.jphotochem.2021.113355
Ghiat I, Saadi A, Bachari K, Coville NJ, Boudjemaa A (2021) Spherical NiCu phyllosilicates photocatalysts for hydrogen generation. Int J Hydrog Energy 46(75):37656–37669. https://doi.org/10.1016/j.ijhydene.2020.10.203
Ghiat I, Boudjemaa A, Bachari K, Coville NJ (2019) Efficient hydrogen generation over a novel ni phyllosilicate photocatalyst. J Photochem Photobiol A Chem 382:111952. https://doi.org/10.1016/j.jphotochem.2019.111952
Tauber A, Schuchmann HP, von Sonntag C (2000) Sonolysis of aqueous 4-nitrophenol at low and high pH. Ultrason Sonochem 7:45–52. https://doi.org/10.1016/S1350-4177(99)00018-8
Ullah S, Ferreira-Neto EP, Pasa AA, Alcântara CC, Acuña JJ, Bilmes SA, Ricci ML, Landers R, Fermino TZ, Rodrigues-Filho UP (2015) Enhanced photocatalytic properties of core@shell SiO2@TiO2 nanoparticles. Appl Catal B Environ 179:333–343. https://doi.org/10.1016/j.apcatb.2015.05.036
Brus LE (1983) A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J Chem Phys 79:5566. https://doi.org/10.1063/1.445676
Anpo M, Shima T, Kodama S, Kubokawa Y (1987) Photocatalytic hydrogenation of CH3CCH with H2O on samll-particle TiO2: size quantization and reaction intermediates. J Phys Chem 91:4305–4310. https://doi.org/10.1002/chin.198743032
Lin H, Huang C, Li W, Ni C, Shah S, Tseng Y (2006) Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl Catal B Environ 68:1–11. https://doi.org/10.1016/j.apcatb.2006.07.018
Ohtani B (2013) Titania photocatalysis beyond recombination: a critical review. Catalysts 3:942–953. https://doi.org/10.3390/catal3040942
Martínez C, Canle LM, Fernández MI, Santaballa JA, Faria J (2011) Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walledcarbon nanotubes—Anatase composites. Appl Catal B Environ 102:563–571. https://doi.org/10.1016/j.apcatb.2010.12.039
Chen Y, Yang S, Wang K, Lou L (2005) Role of primary active species and TiO2 surface characteristic in UV illuminated photodegradation of acid orange 7. J Photochem Photobiol A 172:47–54. https://doi.org/10.1016/j.jphotochem.2004.11.006
Haque M, Muneer M (2007) Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide. J Hazard Mater 145:51. https://doi.org/10.1016/j.jhazmat.2006.10.086
Guettaıa D, Boudjemaa A, Zazoua H, Mokhtarı M, Bacharı K (2018) Enhanced performance of Fe-JUL-15 prepared by ultrasonic method through the photo-degradation of ibuprofen. Environ Prog Sustainable Energy 37:738–745. https://doi.org/10.1002/ep.12748
Saritha P, Raj D, Aparna C, Laxmi P, Himabindu V, Anjaneyulu Y (2009) Degradative oxidation of 2,4,6 trichlorophenol using advanced oxidation processes—a comparative study. Water Air Soil Pollut 200:169–179. https://doi.org/10.1007/s11270-008-9901-y
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177. https://doi.org/10.1016/j.progsolidstchem.2004.08.001
Rao TN, Tryk TN, Fujishima A (2002) In: Bard AJ, Stratmann, M (Eds) Encyclopedia of electrochemistry, semiconductor electrodes and photoelectrochemistry, 6, Wiley, New York, p 540. https://doi.org/10.1021/ja0252678
Bhatkhande DS, Pangarkar VG, Beenackers AACM (2002) Photocatalytic degradation for environmental applications—a review. J Chem Technol Biotechnol 77:102–116. https://doi.org/10.1002/jctb.532
Ahmed S, Rasul MG, Martens WN, Brown R, Hashib MA (2011) Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater. Water Air Soil Pollut 215:3–29. https://doi.org/10.1007/s11270-010-0456-3
Lente G (2015) deterministic kinetics in chemistry and systems biology: the dynamics of complex reaction networks. Springer, Cham, pp 21–59
Herrmann JM, Guillard C, Pichat P (1993) Heterogeneous photocatalysis: an emerging technology for water treatment. Catal Today 17:7–20. https://doi.org/10.1016/0920-5861(93)80003-J
Kitsou I, Panagopoulos P, Maggos Th, Arkas M, Tsetsekou A (2018) Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl Surf Sci 441:223–231. https://doi.org/10.1016/j.apsusc.2018.02.008
Hamad H, Abd El-Latif M, Kashyout AE, Sadik W, Feteha M (2015) Sythesis and characterization of core-shell-shell magnetic (CoFe2O4-SiO2-TiO2) nanocomposites and TiO2 nanoparticles for the evaluation of photocatalytic activity under UV and Visible irradiation. New J Chem 39:3116–3128. https://doi.org/10.1039/C4NJ01821D
Sikong L, Masom P (2013) Photocatalytic reaction of SiO2/TiO2 composite core-shell structured powders. Adv Mater Res 602–604:139–143. https://doi.org/10.4028/www.scientific.net/AMR.602-604.139
Fu N, Ren X-C, Wan J-X (2020) The effect of molar ratios of Ti/Si on core-shell SiO2@TiO2 nanoparticles for photocatalytic applications. J Nanomater. https://doi.org/10.1155/2020/5312376
Lam SM, Sin JC, Lin H, Li H, Lim JW, Zeng H (2020) A Z-scheme WO3 loaded-hexagonal rod-like ZnO/Zn photocatalytic fuel cell for chemical energy recuperation from food wastewater treatment. Appl Surf Sci 514:145945. https://doi.org/10.1016/j.apsusc.2020.145945
Lam SM, Sin JC, Zeng H, Lin H, Li H, Qin Z, Lim JW, Mohamed AR (2021) Z-scheme MoO3 anchored-hexagonal rod like ZnO/Zn photoanode for effective wastewater treatment, copper reduction accompanied with electricity production in sunlight-powered photocatalytic fuel cell. Separ Purif Techn. 265:118495. https://doi.org/10.1016/j.seppur.2021.118495
Liu W, Wang M, Xu C, Chen S, Fu X (2013) Ag3PO4/ZnO: an efficient visible-light-sensitized composite with its application in photocatalytic degradation of Rhodamine B. Mater Res Bull 48:106. https://doi.org/10.1016/j.materresbull.2012.10.015
Acknowledgements
This work is supported by the Directorate General for Scientific Research and Technological Development DGRSDT (Algeria). The DSI-NRF Centre of Excellence in Strong Materials (University of the Witwatersrand, South Africa) is also thanked for their financial support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DG: Conceptualization, Methodology, Writing—original draft. HZ: Visualization, Investigation, Methodology,—review & editing. MRR: Visualization, Investigation, Methodology,—review & editing. KBa: Review & Editing. NJC: Review & Editing. AB: Conceptualization, Writing—review & editing. All the authors commented on previous versions of the manuscript, read and improved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guettaia, D., Zazoua, H., Ramdani, M.R. et al. Removal of ibuprofen using a SiO2@xTiO2 photocatalyst under UV irradiation. Reac Kinet Mech Cat 136, 1085–1106 (2023). https://doi.org/10.1007/s11144-023-02398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02398-9