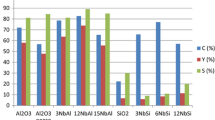
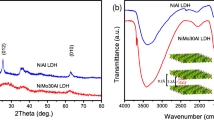
Abstract
Ruthenium containing α-Ni(OH)2 was prepared by a simple impregnation method, the resultant materials with 4 wt% ruthenium loaded nickel hydroxide forms a solid solution of Ni–Ru mixed metal oxide upon calcination. The formation of uniform layered α-nickel hydroxide with ruthenium dispersed on the surface was evident from various analytical and spectroscopic techniques such as powder XRD, XPS, FT-IR and sorption studies. The Ni–Ru-HT catalyst under hydrogen atmosphere showed as a potential catalyst for hydrotreating of anisole with good conversion (90% at 175 °C and 20 bar H2 pressure), with the formation of toluene as a major product along with a considerable amount of methylcyclohexane and benzene as the minor products. The formation of a solid solution of mixed oxide and the dispersion of Ni and Ru on the surface of the oxide enhanced the catalytic activity. Further, the material obtained by calcination at 200 °C is active for the oxidation of cinnamyl alcohol in the presence of tertiary-butyl-hydroperoxide (TBHP) in decane as an oxidant under ambient conditions (90 °C and 6 h). A maximum of 84% conversion of cinnamyl alcohol and more than 80% selectivity for cinnamaldehyde was obtained on the best catalyst. The activity remains intact even after four cycles. The ruthenium on α-Ni(OH)2 was found to be a potential catalyst for the conversion of lignin model compounds to value-added chemicals.














Similar content being viewed by others
References
Sreenavya A, Baskaran T, Ganesh V, Sharma D, Kulal N, Sakthivel A (2018) Framework of ruthenium-containing nickel hydrotalcite-type material: preparation, characterisation, and its catalytic application. RSC Adv 8(44):25248–25257
Ballarin B, Seeber R, Tonelli D, Vaccari A (1999) Electrocatalytic properties of nickel(II) hydrotalcite-type anionic clay: application to methanol and ethanol oxidation. J Electroanal Chem 463(1):123–127
Li M, Wang X, Li S, Wang S, Ma X (2010) Hydrogen production from ethanol steam reforming over nickel based catalyst derived from Ni/Mg/Al hydrotalcite-like compounds. Int J Hydrogen Energy 35(13):6699–6708
Hall DS, Lockwood DJ, Bock C, MacDougall BR (2015) Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proc R Soc A 471(2174):20140792
Baskaran T, Christopher J, Sakthivel A (2015) Progress on layered hydrotalcite (HT) materials as potential support and catalytic materials. RSC Adv 5(120):98853–98875
Zhang J, Chen J, Guo Y, Chen L (2015) Effective upgrade of levulinic acid into γ-valerolactone over an inexpensive and magnetic catalyst derived from hydrotalcite precursor. ACS Sustain Chem Eng 3(8):1708–1714
Manikandan M, Venugopal AK, Prabu K, Jha RK, Thirumalaiswamy R (2016) Role of surface synergistic effect on the performance of Ni-based hydrotalcite catalyst for highly efficient hydrogenation of furfural. J Mol Catal A 417:153–162
Scavetta E, Stipa S, Tonelli D (2007) Electrodeposition of a nickel-based hydrotalcite on Pt nanoparticles for ethanol and glucose sensing. Electrochem Commun 9(12):2838–2842
Sreenavya A, Sahu A, Sakthivel A (2020) Hydrogenation of lignin-derived phenolic compound eugenol over ruthenium-containing nickel hydrotalcite-type materials. Ind Eng Chem Res 59(26):11979–11990
Sahu AK, Dash DK, Mishra K, Mishra SP, Yadav R, Kashyap P (2018) Properties and applications of ruthenium in noble and precious metals-properties nanoscale effects and applications. InTech, London
Cui X, Surkus AE, Junge K, Topf C, Radnik J, Kreyenschulte C, Beller M (2016) Highly selective hydrogenation of arenes using nanostructured ruthenium catalysts modified with a carbon–nitrogen matrix. Nat Commun 7(1):1–8
De S, Zhang J, Luque R, Yan N (2016) Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ Sci 9(11):3314–3347
Zhu C, Cao JP, Zhao XY, Xie T, Zhao M, Wei XY (2019) Bimetallic effects in the catalytic hydrogenolysis of lignin and its model compounds on nickel–ruthenium catalysts. Fuel Process Technol 194:106126
Sreenavya A, Ahammed S, Ramachandran A, Ganesh V, Sakthivel A (2021) Nickel–ruthenium bimetallic species on hydrotalcite support: a potential hydrogenation catalyst. Catal Lett 152(3):848–62
Zhang C, Xing J, Song L, Xin H, Lin S, Xing L, Li X (2014) Aqueous-phase hydrodeoxygenation of lignin monomer eugenol: influence of Si/Al ratio of HZSM-5 on catalytic performances. Catal Today 234:145–152
Khromova SA, Smirnov AA, Bulavchenko OA, Saraev AA, Kaichev VV, Reshetnikov SI, Yakovlev VA (2014) Anisole hydrodeoxygenation over Ni–Cu bimetallic catalysts: the effect of Ni/Cu ratio on selectivity. Appl Catal A 470:261–270
Pichaikaran S, Arumugam P (2016) Vapour phase hydrodeoxygenation of anisole over ruthenium and nickel supported mesoporous aluminosilicate. Green Chem 18(9):2888–2899
Alshehri F, Feral C, Kirkwood K, Jackson SD (2019) Low temperature hydrogenation and hydrodeoxygenation of oxygen-substituted aromatics over Rh/silica: part 1: phenol, anisole and 4-methoxyphenol. Reac Kinet Mech Cat 128(1):23–40
Mallat T, Bodnar Z, Hug P, Baiker A (1995) Selective oxidation of cinnamyl alcohol to cinnamaldehyde with air over Bi–Pt/alumina catalysts. J Catal 153(1):131–143
Niklasson IB, Delaine T, Islam MN, Karlsson R, Luthman K, Karlberg AT (2013) Cinnamyl alcohol oxidizes rapidly upon air exposure. Contact Derm 68(3):129–138
Rucinska E, Miedziak PJ, Pattisson S, Brett GL, Iqbal S, Morgan DJ, Sankar M, Hutchings GJ (2018) Cinnamyl alcohol oxidation using supported bimetallic Au–Pd nanoparticles: an investigation of autoxidation and catalysis. Catal Sci Technol 8(11):2987–2997
Cheng SS, Liu JY, Tsai KH, Chen W, Chang ST (2004) Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem 52(14):4395–4400
Waffel D, Alkan B, Fu Q, Chen YT, Schmidt S, Schulz C, Wiggers H, Muhler M, Peng B (2019) Towards mechanistic understanding of liquid-phase cinnamyl alcohol oxidation with tert-butyl hydroperoxide over noble-metal-free LaCo1–xFexO3 perovskites. ChemPlusChem 84(8):1155–1163
Abad A, Almela C, Corma A, García H (2006) Unique gold chemoselectivity for the aerobic oxidation of allylic alcohols. ChemComm 30:3178–3180
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87(9–10):1051–1069
Nagpure AS, Venugopal AK, Lucas N, Manikandan M, Thirumalaiswamy CS (2015) Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran. Catal Sci Technol 5(3):1463–1472
Varga E, Pusztai P, Ovari L, Oszko A, Erdohelyi A, Papp C, Steinrück HP, Konya Z, Kiss J (2015) Probing the interaction of Rh, Co and bimetallic Rh–Co nanoparticles with the CeO2 support: catalytic materials for alternative energy generation. Phys Chem Chem Phys 17(40):27154–27166
Varga E, Baan K, Samu GF, Erdohelyi A, Oszko A, Konya Z, Kiss J (2016) The effect of Rh on the interaction of Co with Al2O3 and CeO2 supports. Catal Lett 146(9):1800–1807
Sapi A, Halasi G, Kiss J, Dobo DG, Juhasz KL, Kolcsar VJ, Ferencz Z, Vari G, Matolin V, Erdohelyi A, Kukovecz A (2018) In situ DRIFTS and NAP-XPS exploration of the complexity of CO2 hydrogenation over size-controlled Pt nanoparticles supported on mesoporous NiO. J Phys Chem C 122(10):5553–5565
Crisafulli C, Scire S, Minico S, Solarino L (2002) Ni–Ru bimetallic catalysts for the CO2 reforming of methane. Appl Catal A 225(1–2):1–9
Bian Z, Das S, Wai MH, Hongmanorom P, Kawi S (2017) A review on bimetallic nickel-based catalysts for CO2 reforming of methane. ChemPhysChem 18(22):3117–3134
Ramasamy KK, Gray M, Job H, Santosa D, Li XS, Devaraj A, Karkamkar A, Wang Y (2016) Role of calcination temperature on the hydrotalcite derived MgO–Al2O3 in converting ethanol to butanol. Top Catal 59(1):46–54
Jang HW, Urbanek W, Yoo MC, Lee JL (2002) Low-resistant and high-transparent Ru/Ni ohmic contact on p-type GaN. Appl Phys Lett 80(16):2937–2939
Jang HW, Lee JL (2003) Transparent ohmic contacts of oxidized Ru and Ir on p-type GaN. J Appl Phys 93(9):5416–5421
Lee YG, Liao BX, Weng YC (2017) Ruthenium oxide modified nickel electrode for ascorbic acid detection. Chemosphere 173:512–519
Nesbitt HW, Legrand D, Bancroft GM (2000) Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys Chem Miner 27(5):357–366
Grosvenor AP, Biesinger MC, Smart RSC, McIntyre NS (2006) New interpretations of XPS spectra of nickel metal and oxides. Surf Sci 600(9):1771–1779
Acknowledgements
The author thanks DST-SERB-CRG (Project No: CRG/2019/004624) for financial support. Neethu and Sreenavya are grateful to CSIR (File No. 09/1108(0036)/2019-EMR-1) and CUK for the fellowship and Lab facilities. Ashwin is thankful to post metric scholarship-Kerala (e-grantz), India for his fellowship.
Author information
Authors and Affiliations
Contributions
PPN, PA, AS: Methodology, Data curation, Software, Formal analysis, Writing—review and editing. SN, PSA: Data curation, Software, Formal analysis. AS: Conceptualization, Formal analysis, Writing—review and editing, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neethu, P.P., Aswin, P., Sreenavya, A. et al. Ruthenium on α-Ni(OH)2 as potential catalyst for anisole hydrotreating and cinnamyl alcohol oxidation. Reac Kinet Mech Cat 135, 1587–1606 (2022). https://doi.org/10.1007/s11144-022-02211-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02211-z