Abstract
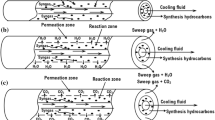
In this study, a mathematical model describing Fischer–Tropsch synthesis over an iron catalyst carried out in two configurations of membrane reactors was developed to predict the process performance. For this purpose, the impact of water removal from the reaction side on syngas conversion and on hydrocarbons selectivity was theoretically analyzed and quantified under different operating conditions. The obtained main results reveal that the process can be intensified when the catalyst was packed in a single region, whereas the produced water was continuously removed from the reaction side to the permeate side, which is constituted of two identical and parallel regions. This configuration design is characterized by a sufficient large area, which can enable fast water removal by an adequate sweep-fluid flow rate. As a result, the conversion and product selectivity could be enhanced obviously at the suitable conditions.






Similar content being viewed by others
Abbreviations
- \(A\) :
-
Cross section (m2)
- \(A_{m}\) :
-
Membrane section (m2)
- \(C_{pg}\) :
-
Specific heat transfer of the gas at constant pressure (J mol−1 K−1)
- \(D\) :
-
Reactor diameter (m)
- \(d_{p}\) :
-
Particle diameter (m)
- \(E_{j}\) :
-
Activation energy for the reaction j (J mol−1)
- \(F_{i}\) :
-
Molar flow rate of component i (mol s−1)
- \(F_{T}^{0}\) :
-
Initial molar flow rate of each component (mol s−1)
- \(F_{T}\) :
-
Total molar flow rate (mol s−1)
- \(J_{{{\text{H}}_{2} {\text{O}}}}\) :
-
Permeation rate of water (mol m−3 s−1)
- \(K_{j}\) :
-
Kinetic rate constant of the FT reaction (mol kg−1 s−1 MPa−1)
- \(K_{WGS}\) :
-
Kinetic rate constant of the WGS reaction (mol kg−1 s−1)
- \(L\) :
-
Reactor length (m)
- \(l\) :
-
Dimensionless reactor length
- \(M\) :
-
Inlet molar flow ratio between hydrogen and carbon monoxide
- \(P_{T}\) :
-
Total pressure (Pa)
- \(P_{i}\) :
-
Partial pressure of component i (Pa)
- \(P_{{{\text{H}}_{2} {\text{O}}}}^{r}\) :
-
Water pressure in reaction side (Pa)
- \(P_{{{\text{H}}_{2} {\text{O}}}}^{p}\) :
-
Water pressure in permeate side (Pa)
- \(Q\) :
-
Volumetric flow rate (m3 s−1)
- \(R_{i}\) :
-
Production rate of component i from the model (mol kg−1 s−1)
- \(R_{j}\) :
-
Rate of reaction j (mol kg−1 s−1)
- \(R_{WGS}\) :
-
Water gas shift reaction rate (mol kg−1 s−1)
- \(r\) :
-
Radius of the reaction zone in MR1 (m)
- \(r_{1}\) :
-
Radius of the permeate zone in MR2 (m)
- \(r_{2}\) :
-
Radius of the MR2 (m)
- \(S_{i}\) :
-
Product selectivity (%)
- \(T\) :
-
Temperature (K)
- \(T_{sh}\) :
-
Shell temperature (K)
- \(U_{sh}\) :
-
Overall heat transfer coefficient shell-fluid (W m−2 K−1)
- \(v\) :
-
Gas velocity (m s−1)
- \(X_{\text{CO}}\) :
-
Carbon monoxide conversion in the reaction j
- \(X_{{{\text{H}}_{2} }}\) :
-
Conversion of syngas
- \(Y_{{{\text{H}}_{2} {\text{O}}}}\) :
-
Removal water
- \(z\) :
-
Axial reactor coordinate
- \(\beta\) :
-
Membrane permeability (mol s−1 m−2 Pa−1)
- \(\mu\) :
-
Gas viscosity (Pa s)
- \(\upsilon\) :
-
Stoichiometric coefficient
- \(\varepsilon\) :
-
Bed porosity
- \(\rho_{g}\) :
-
Gas density (kg m−3)
- \(\rho\) :
-
Catalyst density (kg m−3)
- \(\Delta H_{i}\) :
-
Enthalpy of formation of component i (J mol−1)
- \(g\) :
-
Gas phase
- \(i\) :
-
Component i
- \(j\) :
-
Reaction j
- m :
-
Constant
- n :
-
Constant
- O :
-
Inlet conditions
- sh :
-
Shell side
- FT :
-
Fischer–Tropsch
- FTS :
-
Fischer–Tropsch Synthesis
- HC :
-
Hydrocarbon
- MR1:
-
Membrane reactor with the 1st configuration
- MR2:
-
Membrane reactor with the 2nd configuration
- WGS:
-
Water-gas-shift reaction
References
Kang SH, Bae JW, Woo KJ, Prasad PSS, Jun KW (2010) Fuel Process Technol 91:399–403
Sari A (2014) Chem Eng J 244:317–326
Shin MS, Park N, Park MJ, Cheon JY, Kang JK, Jun KW, Ha KS (2014) Fuel Process Technol 118:235–243
Zolfaghari Z, Tavasoli A, Tabyar S, Pour AN (2014) J Energy Chem 23:57–65
Shimura K, Miyazawa T, Hanaoka T, Hirata S (2014) Appl Catal A Gen 475:1–9
Kwack SH, Park MJ, Bae JW, Ha KS, Jun KW (2011) React Kinet Mech Catal 104:483–502
Pour AN, Khodabandeh H, Izadyar M, Housaindokht MR (2014) React Kinet Mech Catal 111:29–44
Kang SH, Koo HM, Kim AR, Lee DH, Ryu JH, Yoo YD, Bae JW (2013) Fuel Process Technol 109:141–149
Khodakov AY, Chu W, Fongarland P (2007) Chem Rev 107:1692–1744
Fu T, Jiang Y, Lv J, Li Z (2013) Fuel Process Technol 110:141–149
Rohde MP, Schaub G, Khajavi S, Jansen JC, Kapteijn F (2008) Microporous Mesoporous Mater 115:123–136
Dalai AK, Das TK, Chaudhari KV, Jacobs G, Davis BH (2005) Appl Catal A Gen 289:135–142
Espinoza RL, Du Toit E, Santamaria J, Menendez M, Coronas J, Irusta S (2000) Stud Surf Sci Catal 130:389–395
Bayat M, Rahimpour MR, Moghtaderi B (2011) J Nat Gas Sci Eng 3:555–570
Iliuta I, Larachi F, Fongarland P (2010) Ind Eng Chem Res 49:6870–6877
Arabpour M, Rahimpour MR, Iranshahi D, Raeissi S (2012) J Nat Gas Sci Eng 9:209–219
Rahimpour MR, Mirvakili A (2013) J Ind Eng Chem 19:508–522
Marvast A, Sohrabi M, Zarrinpashneh S, Baghmisheh Gh (2005) Chem Eng Technol 28:78–86
Mazzone LCA, Fernandes FAN (2006) Latin Am Appl Res 36:141–148
Fogler HS (2004) Elements of chemical reaction engineering. Prentice-Hall of India, New Delhi
Rahimpour MR, Jokar SM, Jamshidnejad Z (2012) Chem Eng Res Des 90:383–396
Vervloet D, Kapteijn F, Nijenhuis J, van Ommen JR (2013) Catal Today 216:111–116
Wang YN, Xu YY, Xiang HW, Li YW, Zhang BJ (2001) Ind Eng Chem Res 40:4324–4335
Rahmati M, Mehdi M, Soleiman MB (2001) Can J Chem Eng 79:800–804
Rohde MP (2010) In-situ H2O removal via hydrophilic membranes during Fischer–Tropsch and other fuel-related synthesis reaction. KIT Scientific Publishing, Karlsruhe
Fernandes FAN, Teles UM (2007) Fuel Process Technol 88:207–214
Bayat M, Rahimpour MR (2012) J Nat Gas Sci Eng 9:73–85
Fernandes FAN (2007) J Nat Gas Chem 16:107–114
Jager B, Espinoza RL (1995) Catal Today 23:17–28
Feyzi M, Jafari F (2012) Fuel Chem Technol 40:550–557
Jung H, Yang JI, HoonYang J, Lee HT, HyunChun D, Kim HJ (2010) Fuel Process Technol 91:1839–1844
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alihellal, D., Chibane, L. Simulation study of the effect of water removal from Fischer–Tropsch products on the process performance using a hydrophilic membrane reactor. Reac Kinet Mech Cat 117, 605–621 (2016). https://doi.org/10.1007/s11144-015-0961-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0961-x