Abstract
A method for the comparison of kinetic parameters of reaction/process for thermogravimetric measurements at isothermal and dynamic conditions and processes on a larger scale were considered. It is based on the concept of finite time and corresponding total conversion of the solid phase. The method allows for the determination of Arrhenius parameters without the selection of process mechanism. The results obtained for isothermal and dynamic conditions (comparison between dehydration process and thermal decomposition of calcite) indicate that the values of parameter E in both cases are similar, but values of ln A (the entropic factor) differ from each other. It also has been shown that the method of coke and char preparation notably influences the activation energy values of the CO2 gasification reaction, which is associated with varying degrees of devolatilization and corresponding development of the sample pore surface area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the solid phase reactions/processes carried out under high temperature conditions, kinetic expressions take into account the properly determinated rate of the process/reaction (differential form) or after integration—the mass integral g(α), where α represents the fractional conversion of solid.
In the simplest case of an elementary reaction, the kinetic equation takes the form of time–temperature relation, simple to use for the isothermal conditions as given in Eq. (1):
In thermogravimetric analysis, many studies are reported on the thermal stability of solid phase under varying gaseous atmosphere. Regarding the solid fuel pyrolysis and gasification (and heavy liquids as well), the processes are carried out under non-isothermal conditions with a constant heating rate (linear dependence of temperature on time):
The studies performed under isothermal conditions are carried out much less frequently—there is an idea that thermal analysis of condensed phases under these conditions is more reliable and representative of the examination of the thermal dissociation process. Experience indicates that perfect isothermal conditions are not possible to reach in the processes realized at elevated temperatures, even for the most precise and extensive research apparatus initial range is close to the dynamic conditions [1], hence there are often described as quasi-isothermal conditions.
It can be assumed that the initial dynamic conditions have an influence on the further course of the process under isothermal conditions, but we do not have the necessary mathematical apparatus that takes the discussed influence into account in an unambiguous manner.
There are a lot of papers focused on this problem, and certainly serious conflict was initiated by MacCallum and Tanner work published in 1970 [2].
Conversion degree α is not the total differential of time (τ) and temperature (T), because there is their time–temperature relationship (2). The rate of reaction/process as pointed out in [2] takes the following form (assuming that the concept of partial derivative is accepted):
where q = 0 denotes isothermal conditions, q > 0 denotes dynamic conditions according to (2).
Comparative studies on results obtained under isothermal and dynamic conditions as in the papers [3–5] are rarely performed; hence, the dehydration process presented in [4] is a result of numerical simulation of unspecified chemical reaction.
Aim of the work
As this stage is omitted in all considerations given in number of works (e.g. [6–8]), this paper proposes a method that allows for a comparison of kinetic parameters expressed by Arrhenius law (E, A) for processes such as:
carried out under isothermal and dynamic conditions. The method is based on a comparison of finite elements in kinetic terms as complete conversion versus time of reaction/process.
In this aspect, three different reactions and chemical processes have been considered as follows:
-
1.
thermal dissociation of CaCO3 (literature-derived and our own data),
-
2.
thermocatalytic degradation of polyolefins in paraffin oil,
-
3.
course of the Boudouard–Bell reaction (gasification towards CO2).
The object is achieved through the analysis of Eq. 3 in the versions given in the literature, i.e.:
-
(a)
as a general integral form in relation only to temperature according to Błażejowski [8],
-
(b)
differential equation according to Šesták et al. [3, 9, 10], which was proposed in advance by Błażejowski [8] as a category of reaction rate dα/dτ.
Comparisons of integral kinetic forms is recommended. Finally, this work uses the concept of finite time in integral category \( \int_{{\tau_{i} }}^{{\tau_{f} }} {d\tau = \tau_{f} - \tau_{i} = \tau } \), i.e. τ is the time of reaction/process.
Analysis of the equations given in the literature
Solution of Eq. 3 through temperature criterion according to [8]
Based on Błażejowski considerations [8], representing time–temperature relationship 3 within the limits of integration of [0, T], the following mass integral g(α) can be presented as a function of the temperature only (Eq. (11) in [8], for exponent b = 0):
where according to definitions:
The solution of Eq. 4 is described in detail in [8] (Appendix A) and can be written in form
So the aim of this work is propose another way to obtain solution 6, the same as was given in [8].
Particular partial derivatives resulting from Eq. 1 are as follows:
After introducing after applying definition (5), we obtain an expression which is in its final form equivalent to Eq. 4:
In the second integral of Eq. 9, as the subintegral expression 1 can be used, we obtain a notation as follows:
After differentiation, the following expression is obtained:
Equation 11 is a linear differential equation, ordinary with non-separated variables, which can be solved by primary assumption that the first term on the right hand side in Eq. 11 equals 0. Hence, Eq. 11 can be rewritten in a simplified form by means of Eq. 12:
and finally the following equation can be obtained when C(T) is not specified:
Nevertheless, the expression for C(T) is temperature-dependent only, so after solving the differential equation, it can be shown that:
and finally we obtain Eq. 13, and thus Eq. 4 in the form of Eq. 6.
Using Eq. 2 as \( \tau = \frac{{\left( {T - T_{i} } \right)}}{q} \), then the notation (6) is a classical general form of integral kinetic equations: g(α) = kτ.
In a margin of this discussion for the small values of conversion degree, functions expressing the mass integrals in Eq. 13 can be expanded in series and retain it on the first term (g(α) = α [11]), then we obtain the Szarawara–Kozik temperature criterion [12] written as:
which allows to estimate the approximate value of the activation energy for \( 0 < \alpha < \approx 0.2 \). These considerations confirm the correctness and importance of relationship ln α or more precisely \( \ln g(\alpha )\;{\text{versus}}\;1/T \) in chemical kinetics.
Other solutions resulting from the differential Eq. 3
The following equation proposed by Šesták et al. is starting point for comparison of kinetic effects in isothermal, recognized as isothermal (quasi-isothermal) and dynamic methods [3, 9, 10] and Błażejowski [8] (Eq. (15) in paper [8]):
Equation 16 can also be obtained by the differentiation of solution (6) with respect to time, when T is the linear function of time [8].
The authors confirm mathematical correctness of Eq. 16 but also raises doubts about its physicochemical sense.
Isothermal measurements T = Ti
Equation 16 is transformed into a well-known equation in the category of conversion degree \( \alpha \;{\text{versus}}\;{\text{time}}\;(\tau ) \):
and further—analogously to Eq. 1:
Dynamic measurements and field of temperature implementation (1), T ≥ Ti
Substituting time in Eq. 16 by increasing temperature according to notation (1), we obtain a kinetic equation in categories \( \alpha \;{\text{versus}}\;{\text{temperature}}\,(T) \):
Solution of Eq. 19
Equation 19 is written as Eq. 4 containing the Arrhenius law by means of mass integrals g(α) and integration constant C:
Individual be rewritten in a symbolic way:
Substituting the solution of particular integrals to Eq. 21 (Appendix: Eqs. 28 and 30) the factor containing non-elementary integral J (Eq. 29) is reduced, also appearing in notation (4) and (10).
Finally, we obtain:
For all the known g(α) functions (Table 1) if T = T then g(α = 0) = 0 and integration constant C = 0.
Finally, Eq. 22 assumes the same form as well-known Eq. 18 because from notation (1) follows that T = T i + qτ.
Finite time method (FTM)
The term of “finite time” means total reaction time corresponding to the conversion Δα = α = 1:
-
(a)
for isothermal conditions as a time of complete reaction,
-
(b)
for dynamic conditions as a time corresponding to increase temperature (1) from T = T i (α = 0) to T = T f , where T f corresponds to conversion degree α = 1.
We use Eq. 22 for the constant of integration C = 0 and for the condition:
takes a form:
Finally, we obtain the following relation valid for isothermal measurements carried out at several different temperatures \( \left( {T = T_{i} = T_{f} = {\text{i}}\;{\text{d}}\;{\text{e}}\;{\text{m}}} \right): \)
where τ means time of the process, and T f isothermal temperature, and for dynamic conditions:
The structure of Eq. 25, especially when T i = 0 K (as usually used in thermokinetic equations for dynamic conditions [3–10, 12]), reminds the Kissinger law in a version presented in [13, 14], except that the temperature of maximal rate of reaction (degassing) T m is replaced by T f . Thus, Eq. 25 may be classified as an isoconversional method.
In these considerations, the tedious step of determining the function f(α) or g(α) is omitted, assuming that g(α = 1) = 1, although it is not true relation for all kinetic functions. But in this case, the starting and ending state is important in a kinetic sense.
There are works that use relation −ln τ versus 1/T, but it is a variant of isoconversional methods (for the assumed values of conversion degree α) [15–17].
Literature data—dehydration process
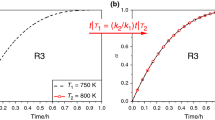
The finite time method was used to analyze simulated dehydration process under isothermal (five different temperatures) and dynamic conditions (five different heating rates) [4]. Based on these data, kinetic parameters were calculated according relations 24 and 25 as shown in Fig. 1, and compared with the values given by the authors in Table 2. Literature and calculated values of kinetic parameters are very similar, although the proposed approach allows to avoid cumbersome procedure of f(α) or g(α) determination. Therefore, it can be assumed that the proposed methodology can be a reliable in order to determine the kinetic parameters of simple reaction.
Experimental
-
i.
Studies of the thermal dissociation of a model compound CaCO3 (analytically pure, POCh Gliwice) were performed in MOM Q 1500D. Analyses were carried out in the temperature range of 20–1,000 °C, with a heating rate varying from 2.5 to 20 K min−1, for sample masses up to 200 mg and the flow of N2 to 250 cm3 min−1. The results of the analysis were recorded by the digitizer.
-
ii.
Studies of the thermal decomposition of polyethylene with paraffin oil (2:1) were performed in Mettler Toledo TGA/SDTA-851 analyzer. Analyses were carried out in the temperature range of 25–1,000 °C at a heating rate from 1.5 to 24 K min−1, for sample masses of about 15 mg and the flow of N2 up to 100 cm3 min−1. The process of the thermal decomposition of polyethylene samples into paraffin oil was also analyzed on a laboratory scale. The sample weight was set to 50 g, and evolved volatile products were condensed in a water cooler. Due to the lack of weighing of the reactor during the process, the amount of volatile products was adopted as a measure of conversion degree. The process was carried out under dynamic conditions with average heating rate ranging from 5 to 9 K min−1 until complete conversion was reached. Cement and microcrystalline silica were used as an inorganic additives in the process.
-
iii.
Studies of chars derived from the slow pyrolysis of coal was performed in MOM Q 1500D under quasi-isothermal conditions. Analyses were carried out in the temperature range of 800–1,000 °C for sample masses up to 200 mg and the flow of N2 set to 250 cm3 min−1. The results of the analysis were recorded by the digitizer. For the same sample, and three others (smokeless fuel, semi-coke, blast furnace coke) studies were conducted in an apparatus based on the method proposed in 1955 by Dahme and Junker after modification. The measurements were carried out under the following conditions: sample weight: 10 g; grinding 1–3 mm. Samples were dried at 150 °C for 2 h. Processes were performed at 850–1,050 °C to particular conversion of sample. The flow of CO2 amounted 2.5 cm3 s−1.
Results
Thermal decomposition of calcium carbonate
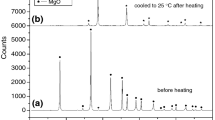
The next stage of the verification of the proposed methodology in order to obtain kinetic parameters according to Eqs. 24 and 25 was to analyze our own experimental data and experimental data provided by other authors [5, 18]. The TG curves for CaCO3 are shown in Fig. 2.
Characteristic temperatures and times of the process for CaCO3 thermal decomposition determined on the basis of our own experimental data are shown in Tables 3 and 4.
In [5], the thermal decomposition of CaCO3 was studied for 6 different heating rates under dynamic conditions (in the range of 1–15 K min−1) and at seven temperatures under isothermal conditions (in the range 973–1,073 K). However, as was presented in [18], the thermal decomposition of CaCO3 (sample mass 10 mg) was analyzed. At nine heating rates under dynamic conditions (in the range 0.2–36 K min−1), but not analyzed under isothermal conditions. Based on Eqs. 24 and 25, kinetic parameters were calculated for our own experimental data and data from other researchers [5, 18], and the results are shown in Table 5 and Fig. 3, correspondingly.
The comparison of the values is summarized in Table 5 and substantially confirms that the proposed methodology for determining the kinetic constants based on Eqs. 24 and 25 by giving similar results despite the differences in mass of samples. It seems to be also important that the procedure for the determination of kinetic parameters is simplified without the selection of mechanism f(α) or g(α), which can lead to divergent values of kinetic parameters. Technically, FTM is not appropriate to describe the kinetics of F1 (see Table 1), but the obtained results indicate that it is also useful for such cases. Fig. 3 shows our own experimental data for the process of thermal decomposition of calcium carbonate combined with literature data [5, 18]. The experimental data can be linearized with a high value of r 2 coefficient along nearly parallel lines—for a dynamic and isothermal conditions. Hence, similar values of activation energy were obtained, and mutual shift is a consequence of difference in value of entropic factors, whose values are higher for the tests performed under isothermal conditions. The results show quite clearly that a finite time method can be successfully used to compare the kinetic constants obtained by studies under dynamic and isothermal conditions.
For data obtained under dynamic conditions, values of kinetic parameters using the Flynn–Wall–Ozawa method (FWO) based on relation 19 were also determined:
and compared with obtained previously. The values of E calculated by means of FWO are higher than for the finite time method, and values reported in the literature [18] as well.
Thermal decomposition of polyethylene–paraffin oil mixtures
The thermal or thermo-catalytic decomposition of polyolefins and their mixtures with low molecular organic compounds is an interesting method of recycling, especially for contaminated and mixed fractions. Hence it is important to study their thermal properties and thermogravimetric studies of polyethylene LDPE–paraffin oil (2:1) mixtures were performed. TG curves are shown in Fig. 4.
Two stages of mass loss are clearly noticeable on TG curves under the dynamic conditions. The first of them is associated with the evaporation and partial decomposition of paraffin oil, while decomposition the polymer occurs in the second one. The values of characteristic temperatures for the both steps are listed in Table 6.
The results for particular steps are shown in Table 7 and compared with the values obtained for the process of thermal decomposition carried out on the laboratory scale (50 g) Fig. 5. The conversion degree obtained on the laboratory scale is different from the TGA tests, but FTM allows to compare them because the end of the solid phase conversion corresponds to the end of volatile products release. It is evident that the process on the laboratory scale is similar to the first stage of TGA (but does not become single stage, it is rather combination of two stages observed for TGA experiments). Additionally, the process on larger scale proceeds with lower E, which is characterized by catalytic processes. Changing the scale and FTM offer new opportunities for assessment and factorial interactions.
The results of thermal decomposition of LDPE–paraffin oil mixtures in the form of Eq. 25
The effect of inorganic additives in the thermal decomposition of polyethylene was also studied, and an analysis was performed using Eq. 25. It was observed that the relation ln(q/(T f − T i )) versus 1/T f clearly indicates the catalytic influence of the tested materials by a decrease in activation energy in the second stage of decomposition (LDPE), which is especially evident in the case of microcrystalline silica (decrease from 265.3 to 193.5 kJ/mol) as shown in Fig. 6.
The results of II stage of thermal decomposition of LDPE–paraffin oil mixtures with addition of inorganic additives by Eq. 25
Chars and cokes
The gasification processes of carbonaceous materials of different origin in compliance to Boudouard–Bell (gasification by carbon dioxide) reaction are characterized in the literature with estimation of Arrhenius equation parameters for the specified forms of kinetic equations. Regarding only the variation of activation energy, it is generally found tho range from 100 to 250 kJ/mol depending on fuel rank. Additionally, for non-volatile graphite it is given as 280.3 kJ/mol [20]. The most common case is when the specific value of activation energy is dependent on the content of volatile matter in the raw material VMdaf. Therefore, when the value of activation energy is given, the second parameter of Arrhenius relationship (pre-exponential factor, A) can be estimated. In order to find the applicability of the finite time method for studies on the reactivity of solid fuels towards CO2 appropriate calculations were performed by using own and literature-derived data.
Data on CO2 gasification rate were collected for the following carbonaceous materials:
-
chars derived from sub- and bituminous coal of different origins (II–IV, VII, XI–XIV),
-
biomass (pine) chars (IX and X), asphaltite char (VI), waste tire char (VIII),
-
petroleum coke (V),
-
carbon black (I).
Besides their origin, the listed materials differ distinctly in the devolatilization conditions under which they were prepared. In each case, rates of gasification reaction were investigated with TGA, carried out under isothermal conditions for wide range of temperatures from 775 to 1,400 °C. Special attention must be paid to a fact that studies on the CO2 gasification of solid fuels performed under dynamic conditions are very rare.
According to the variation of carbon conversion degree with time presented in each paper, values of the finite time were read. In the case of relationships showing incomplete conversion, the values of reaction time were extrapolated. Subsequently, values of activation energy and pre-exponential factor were estimated, using Eq. 24, as presented for each sample in Fig. 7. The obtained values of activation energy were compared with those presented in each paper in Table 8. As can be seen, values of activation energy estimated with the finite time method are comparable to those found in result of a full kinetic analysis performed by authors of cited papers.
Results of studies on gasification of different samples with CO2 in isothermal conditions in functional scales (Eq. 24), samples no. XV–XIX refer to our own experimental data
For comparison, studies were performed on a bigger scale of 10 g of a sample under the quasi-isothermal conditions (for temperature in range of 850–1,050 °C) and under atmospheric pressure for sample nos.: XV–XVIII as shown in Table 8 and Fig. 7. Studied samples differ significantly with their origin and application:
-
smokeless fuel—sample no. XV,
-
semi-coke—sample no. XVI,
-
blast furnace coke, produced in one of Polish coke plants—sample no. XVII,
-
char derived from pyrolysis of Polish steam coal—sample no. XVIII (rate of gasification with CO2 of this sample was also examined with TGA method, according to the methodology previously described in “Experimental” section; sample no. XIX in Table 8 and Fig. 7)
Values of finite time were determined by means of analyzing the relationship between conversion degree and time, when the relationship represented the complete conversion of carbon, the time of reaction was found, otherwise according to the shape of the α versus time relationship, corresponding values were extrapolated.
During the analysis, relationships between values of reaction time and temperature of process by means of Eq. 24, which is presented in Fig. 7, two areas were noticed which are separated close to point “P” as a diffusion-controlled area observed at higher temperatures (left-hand side) and a kinetically controlled area (right-hand side). The latter can be distinguished according to reactivity of the samples for:
-
representing samples of low reactivity, which activation energy was higher than 150 kJ mol−1,
-
representing samples of higher reactivity, which activation energy is lower than 150 kJ mol−1,
A schematic diagram representing the above mentioned relationships is given in the conclusion no. VII.
As shown in Fig. 7, a trend line for intermediate reactivity is represented by several of the studied samples. In turn, our own series of measurements are located in the area representing samples having lower and moderate reactivity.
At the same time, values of activation energy estimated by means of fitting Eq. 24 to our own experimental data are also given in Table 8, for comparison with values obtained using literature-derived data. All data comprising Table 8 refer to isothermal conditions, which are preferred for studies on the reactivity of solid fuels.
Data given in Table 8 indicate that values of activation energy of CO2-gasification reaction increase when final temperature of pyrolysis increases (comparing samples nos. XI and XII, as well as XIII and XIV). Values of activation energy are also higher for chars obtained via slow pyrolysis rather than rapid pyrolysis, which is related to the higher extent of devolatilization of carbonaceous materials and in result, with higher evolution of the surface area. On the other hand, the activation energy is less dependent on the nature and rank of parent carbonaceous material.
For many chemical reactions, a linear relationship between Arrhenius parameters is observed. Such a phenomenon is called the compensation effect (KCE). Many authors distinguish the KCE from an isokinetic effect, where an intersection of Arrhenius relations: ln k versus 1/T for one coordinate (in one point) is observed. As a result, an isokinetic temperature can be calculated. In practical experimental investigations, interception of Arrhenius relationships is noticed in some temperature range, what is designated as a “false” compensation effect [30, 31]. Due to the mentioned blurring effect of interception point, some authors suggest to name such a phenomenon as “diffused isokinetic effect”. Occurrence of this kind of relationships can be associated with random experimental and systematic errors [30, 32, 33].
Values of activation energy, as well as pre-exponential factor obtained via analysis of Eq. 24 for literature-derived and our own experimental data (except for the data for sewage sludge) are presented in analytical form of relation ln A versus E in Fig. 8 and described by relation (A in min−1, E in kJ/mol):
where \( k_{iso} = 0.03341\min^{ - 1} \) and T iso = 1,316.4 K.
As can be seen in Fig. 8, the calculated equation representing the compensation effect reflects the additional data for gasification of coal performed in fluidized bed reactor, FBR [34] very well. The data for FBR were obtained during series of experiments and the kinetics was studied on the basis of gas evolution rate. Values of ln A and E were derived after the analysis of F1 kinetics, although that g(α = 1) = ∞ (see Table 1, Eqs. 22 and 23) satisfying Eq. (27) very well. For comparison, data obtained for sewage sludge samples gasified under dynamic conditions have been added to Fig. 8 [35]. Values of A and E for sewage sludge were calculated according to the Kissinger relationship for four heating rates and for the temperature range corresponding to the CO2 gasification reaction.
Analysis of Fig. 8 indicates that the isokinetic effect can be observed for the literature data. Our own experimental data seem to create another relationship. However, it is still possible to depict all samples with one relationship. At the same time, the relation of ln A versus E for sewage sludge samples does not represent the trendline determined for other samples. This diversification can be caused by complex characteristics of sewage sludge samples and by dynamic conditions, in which experiments were performed. It should be noted here that other results presented in Fig. 8 were obtained in experiments carried out in dynamic conditions.
Generally, it is accepted that reactivity towards CO2 is strictly connected with a rank of carbonaceous material. The rank of materials can be expressed with several parameters, i.e. content of volatile matter. As can be seen in Fig. 9, a correlation between activation energy (considered as a parameter expressing the reactivity, calculated with the finite time method) and content of volatiles has been found. Data presented in Fig. 9 have been narrowed to more devolatilized chars after excluding the outcomes for high volatile biomass chars. Taking into account the full range of data could result in a lack of satisfactory correlation. Hence, it is more justified to divide the data into groups or families of materials being similar with their origin. As shown in Fig. 9, considerably better correlation has been found for the family of chars derived from Chinese coals [22, 29].
The relationships established between activation energy and content of volatile matter can be used for approximation of the values of Arrhenius parameters. For this purpose, also the relationship for KCE, given by Eq. 27, should be taken into consideration. This approach has potentially great technological significance because allows for prediction the reactivity of fuels when only the results of proximate analysis are known.
Discussion
A comparison of kinetic parameters for solid phase reactions/processes performed under different conditions is feasible, when the kinetic notion of finite time is assumed. This term is generally applied, e.g. for iterative processes, for simulations of deformations, stresses and displacements, whereas it is not considered as a fundamental concept in chemical kinetics according to IUPAC. Nevertheless, for a significant number of physicochemical processes, the notion of finite time can be found [36]. It has been stated in this work that the finite time can be defined via integral categories as τ, for which complete conversion Δα = 1 is observed.
Thus, for the for comparison, Eq. 24 is applied for isothermal conditions, and Eq. 25 is for dynamic conditions.
From several papers [4, 17, 37–39] (e.g. Eq. 3.6 in [17]), it follows that kinetic equations are successfully employed for the interpretation of kinetics under isothermal conditions in the form of the following relation:
and for dynamic conditions as well, for q > 0, where Eq. 25 is expressed as the modified Kissinger law, taking into account temperature of maximum weight loss T m in form as presented below [16, 17, 40]:
In this paper, one step further has been taken, assuming finite course of reaction/process. Furthermore, it can be admitted that this approach eliminates some drawbacks of technological usability of proposed isoconversional methods based on the Kissinger law.
If we compare the analysis of experimental data for CaCO3 by methods based on the temperature of maximum weight loss T m (e.g. the Kissinger and Augis–Bennet method [40]) with the finite time method, we can conclude that there are linear correlations between left- and right-hand side of the particular relations, and as a consequence, there is a possibility to find a relation between values of kinetic parameters. It should be noted here that left-hand side of the kinetic equation (relation Tf − Ti) is always occurring, applying for variability of kinetic analysis conditions (isothermal Eq. 24, dynamic Eq. 25) and across a wide range of scales.
Furthermore, using FTM for the right-hand side of the equation is logical, and for isothermal conditions the temperature (T) corresponds to Tf characteristic for non-isothermal conditions, and also it is feasible to use (Tm) but mainly for dynamic conditions.
In the case of the analysis of model compounds (dehydration reaction with simulated data [4], and the thermal dissociation of calcite with our own and literature-derived data [5, 18]), in the latter case, it was found that, compared to quasi-isothermal for similar values of activation energy, dynamic conditions give lower values of pre-exponential factor in the Arrhenius relationship, for which meaning of an entropic factor is assigned [31].
In the case of studies on the decomposition of selected polyolefins in technological oil, the course of micro-scale process carried out under dynamic conditions was compared to the process performed under isothermal conditions on a laboratory scale (15 mg and 100 g). Values of E estimated for laboratory conditions are lower than for the decomposition on the TGA scale for the stage corresponding to the decomposition of the selected polymer, which means that the process on the laboratory scale is coming to the course of oil decomposition, but in fact it is a combination of both stages observed in micro-scale studies.
However, the most satisfactory results have been obtained by comparing literature-derived and our own data on the course of the Boudouard–Bell reaction (C + CO2 = 2CO). For this reaction, a comparison has been made between the course of micro-scale reaction under quasi-isothermal conditions (>200 mg) and the course of the process performed on a scale of 10 g in experimental set-up described in detail in the Experimental. In those cases, both the earlier and currently performed studies did not give satisfactory results for the micro-scale CO2 gasification process under dynamic conditions, at least for as high a conversion degree as was obtained in paper [42] for Shivee Ovoo coal.
Therefore, the performed comparison applies only to isothermal conditions, in spite of considerable enlargement of scale, and all studied carbonaceous materials differ with their rank and carbonization degree. This fact has a great significance for analysis of the Boudouard–Bell reaction, which proceeds right after the pyrolysis stage, which was presented in our own paper [43].
Conclusions
-
i.
The proposed method is applicable in every case when complete conversion of condensed phase occurs (Δα = 1). It allows the comparison of kinetic parameters of chemical reactions/processes, both under isothermal and dynamic conditions (for polyisothermal measurements and in different heating rates, respectively), as well as for comparison of processes performed on different scales, and influence of catalysts. If one can divide the process that can be described by g(α) function then Eq. 1 is fundamental and can be finally expressed for the isothermal (Eq. 24) and dynamic (Eq. 25) measurements. In this method, the parameters of Arrhenius relations can be estimated without the determination of solid phase conversion degree (α) and f(α) as well. The kinetic triplet is therefore reduced to two parameters: E − A (or E − lnA [41]).
-
ii.
Results obtained for isothermal and dynamic conditions (comparison for dehydration process and dissociation of calcite) indicate the correctness of proposed method, because the values of parameter E in both cases are similar, whereas values of ln A (entropy factor) are greater for experiments performed in isothermal conditions.
-
iii.
A comparison has been also made between thermal decomposition of complex polyethylene-oil mixtures under dynamic conditions on scale of 15 mg (TGA) and the process performed on a laboratory scale (sample mass ~100 g). It was also observed that relation 25 clearly indicates the catalytic influence of tested materials by decreasing of activation energy in the second decomposition stage (LDPE).
-
iv.
Studies on CO2 gasification of solid fuels performed under isothermal conditions are much more common. Hence, comparison has been made for processes carried out isothermally for wide range of carbonaceous materials, for literature-derived and our own data. It has been pointed out that values of activation energy for CO2 gasification increase when the temperature of pyrolysis increases (comparison of samples nos. XI and XII, and XIII and XIV in Table 8) and are greater for samples derived via slow rather than rapid pyrolysis, which is connected with different extent of devolatilization and hence—different pore surface area development. For all analyzed samples, the kinetic compensation effect KCE was found (Eq. 27). Hence, KCE relation seems to be correct and convenient way to kinetic characterization of condensed phase conversion independently on the measurement conditions.
Abbreviations
- A:
-
Pre-exponential constant in Arrhenius equation (min−1)
- A, B, C:
-
Substrates and product of reaction
- α :
-
Conversion degree, 0 ≤ α ≤ 1
- C:
-
Constant of integration
- C(T):
-
Expression depending on temperature
- E:
-
Activation energy (J mol−1)
- f(α), g(α):
-
Functions depending on the reaction mechanism
- J, J1, J2 :
-
Integrals
- k:
-
Reaction rate constant (min−1)
- ν :
-
Stoichiometric coefficient
- q :
-
Heating rate (K min−1)
- R = 8.314 J mol−1 K−1 :
-
Universal gas constant
- r 2 :
-
Determination coefficient
- T :
-
Temperature (K)
- τ :
-
Time (min)
- VM daf :
-
Volatile matter, dry ash-free state (%)
- i :
-
Initial state
- j :
-
Symbol of coordinate
- f :
-
Final
- g :
-
Gaseous
- m :
-
Maximal
- s :
-
Solid
References
Mianowski A, Bigda R, Zymla V (2006) Study on kinetics of combustion of brick-shaped carbonaceous materials. J Thermal Anal Calorim 84:563–574
MacCallum JP, Tanner J (1970) Derivation of rate equations used in thermogravimetry. Nature 225:1127–1128
Holba P, Šesták J (1972) Kinetics with regard to the equilibrium of processes studied by non-isothermal techniques. Z Phys Chem Neue Folge 80:1–20
Khawam A, Flanagan DR (2006) Basics and applications of solid-state kinetics: a pharmaceutical perspective. J Pharm Sci 95:472–498
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Li C-R, Tong B, Tang R, Málek J, Mitsuhashi T (2000) Computational aspects of kinetic analysis. Part A: the ICTAC kinetics project-data. Methods and results. Thermochim Acta 355:125–143
Šesták J, Šatava V, Wendlandt WW (1973) The study of heterogeneous processes by thermal analysis. Thermochim Acta 7:447–504
Vyazovkin S, Wight CA (1997) Kinetics in solids. Annu Rev Phys Chem 48:125–149
Błażejowski J (1981) Evaluation of kinetic constants for the solid state reactions under linear temperature increase conditions. Thermochim Acta 48:109–124
Šesták J (1982) Mĕřerení termofyzikálních vlastností pevných látek. Academia, Praha, p 175
Šesták J (2004) Heat, thermal analysis and society. Nucleus HK®, Hradec Králové, p 147
Mianowski A (2000) Thermal dissociation in dynamic conditions by modeling thermogravimetric curves using the logarithm conversion degree. J Thermal Anal Calorim 59:747–762
Mianowski A, Radko T (1994) The possibility of identification of activation energy by means of the temperature criterion. Thermochim Acta 247:389–405
Mianowski A (2003) The Kissinger law and isokinetic effect. Part I. Most common solutions of thermokinetic equations. J Thermal Anal Calorim 74:953–973
Mianowski A, Bigda R (2004) The Kissinger law and isokinetic effect. Part II. Experimental analysis. J Thermal Anal Calorim 75:355–372
Reading M, Dollimore D, Rouquerol J, Rouquerol F (1984) The measurement of meaningful activation energies. J Therm Anal 29:775–785
Budrugeac P (2011) Critical considerations on the Augis and Bennett method for evaluating the crystallization activation energy by means of non-isothermal data. J Non-Cryst Solids 357:1101–1105
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Mianowski A, Baraniec I (2009) Three-parametric equation in evaluation of thermal dissociation of reference compound. J Thermal Anal Calorim 96:179–187
Ozawa T (1986) Non-isothermal kinetics and generalized time. Thermochim Acta 100:109–113
Marchon B, Tysoe WT, Carrazza J, Heinemann H, Somorjai GA (1988) Reactive and kinetic properties of carbon monoxide and carbon dioxide on a graphite surface. J Phys Chem 92:5744–5749
Rao YK, Jalan BP (1972) A study of the rates of carbon–carbon dioxide reactions in the temperature range 839°C to 1050°C. Metall Trans 3:2465–2477
Liu TF, Fang Y-T, Wang Y (2008) An experimental investigation into gasification reactivity of chars prepared at high temperatures. Fuel 87:460–466
Zou JH, Zhou ZJ, Wang FC, Zhang W, Hua Dai Z, Liu HF, Yu ZH (2007) Modeling reaction kinetics of petroleum coke gasification with CO2. Chem Eng Process 46:630–636
Fouga GG, De Micco G, Bohe AE (2011) Kinetic study of Argentinean asphaltite gasification using carbon dioxide as gasifying agent. Fuel 90:674–680
Everson RC, Neomagus HWJP, Kaitano R, Falcon R, du Cann VM (2008) Properties of high ash coal-char particles derived from inertinite-rich coal. II. Gasification kinetics with carbon dioxide. Fuel 87:3403–3408
Murillo R, Navarro MV, Lopez JM, Aylon E, Callen MS, Garcia T, Mastral AM (2004) Kinetic model comparison for waste tire char reaction with CO2. Ind Eng Chem Res 43:7768–7773
Cetin E, Moghtaderi B, Gupta R, Wall TF (2005) Biomass gasification kinetics: influences of pressure and char structure. Compos Sci Technol 177:765–791
Fermoso J, Stevanov C, Moghtaderi B, Arias B, Pevida C, Plaza MG, Rubiera F, Pis JJ (2009) High-pressure gasification reactivity of biomass chars produced at different temperatures. J Anal Appl Pyrolysis 85:287–293
Wu S, Gu J, Zhang X, Wu Y, Gao J (2008) Variation of carbon crystalline structures and CO2 gasification reactivity of Shenfu coal chars at elevated temperatures. Energy Fuels 22:199–206
Shenrik AN (1998) Means of checking for false compensation effects in liquid-phase calculations. Theor Exp Chem 34:211–217
Liu L, Guo Q-X (2001) Isokinetic relationship. Isoequilibrium relationship and enthalpy–entropy compensation. Chem Rev 10:673–695
Berrie PJ (2012) The mathematical origins of the kinetic compensation effect: 1. The effect of random experimental errors. Phys Chem Chem Phys 14:318–326
Berrie PJ (2012) The mathematical origins of the kinetic compensation effect: 2. The effect of systematic errors. Phys Chem Chem Phys 14:327–336
Kodama T, Funatoh A, Shimizu K, Kitayama Y (2001) Kinetics of metal oxide-catalyzed CO2 gasification of coal in a fluidized-bed reactor for solar thermochemical process. Energy Fuels 15:1200–1206
Nowicki L, Bedyk T, Stolarek P, Ledakowicz S (2008) Thermo-chemical processing of sewage sludge under different gaseous atmosphere. European Conference on Sludge Management, ECSM 08, 1–2 September 2008. Book of abstracts. University of Liege, Belgium, p 58
Arnone A, Sestini A (1991) Multigrid heat transfer calculations using different iterative schemes. Heat Transf B Fundam 19:1–11
Andersen B (1984) Thermodynamics for processes in finite time. Acc Chem Res 17:266–271
Reading M, Dollimore D, Rouquerol J, Rouquerol F (1984) The measurement of meaningful activation energies. J Thermal Anal 29:775–785
Rodante F, Vecchio S, Tomassetti (2002) Kinetic analysis of thermal decomposition for penicillin sodium salts. J Pharm Biomed Anal 29:1031–1043
Augis JA, Bennett JE (1978) Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Thermal Anal 13:283–292
Brill TB, Gongwer PE, Williams GK (1994) Thermal decomposition of energetic materials. 66. Kinetic compensation effects in HMX, RDX and NTO. J Phys Chem 98:12242–12247
Avid B, Purevsuren B, Born M, Dugarjav J, Ya D, Tuvshinjargal A (2002) Pyrolysis and TG analysis of Shivee Ovoo coal from Mongolia. J Thermal Anal Calorim 68:877–885
Mianowski A, Siudyga T (2012) Analysis of relative rate of reaction/process. J Thermal Anal Calorim 109:751–762
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Mianowski A, Radko T (1992) Evaluation of the solutions of standard kinetic equation for non-isothermal conditions. Thermochim Acta 204:281–293
Órfão JJM (2007) Review and evaluation of the approximations to the temperature integral. AIChE 53:2905–2915
Acknowledgments
This study was partly performed within a framework of the Strategic Programme: “Advanced Technologies for Energy Generation: Development of Coal Gasification Technology for High-Efficient Fuels Production and Energy Generation” No. SP/E/3/7708/10. The financial support from Polish National Centre for Research and Development is gratefully acknowledged. M. Tomaszewicz has received a grant under the project “DoktoRIS—Scholarship programme for innovative Silesia” co-financed by the European Union under the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
In notation (21), only the J 2 integral represents analytical form of an elementary function (the constant of integration is omitted):
The remaining two expressions (J and J 1) are approximations as follows:
Generally, it is well known that the reliability of Arrhenius equation parameters depends on the accuracy of the analytical form of the approximation temperature integral (also denoted as a Arrhenius-type) (Eq. 29). The first approximation denoted as Coats–Redfern [44] is represented with a form as follows:
in general, the expression \( \left( {1 - 2\frac{RT}{E}} \right) \) is neglected.
The solution consisting of notation in a form of power series comprises of notation 31 was given in the paper [45]
where
However there are many others papers, e.g. [40], the most comprehensive study on this scope was given in the paper [46].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mianowski, A., Tomaszewicz, M., Siudyga, T. et al. Estimation of kinetic parameters based on finite time of reaction/process: thermogravimetric studies at isothermal and dynamic conditions. Reac Kinet Mech Cat 111, 45–69 (2014). https://doi.org/10.1007/s11144-013-0613-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0613-y