Abstract
Purpose
Patient-reported outcomes (PROs) provide the patient’s perspective of the impact of treatment. Evidence suggests that PRO content of randomised controlled trials (RCTs) protocols is generally sub-optimal. This study aimed to describe and evaluate the PRO-specific content of ovarian cancer RCT protocols.
Methods
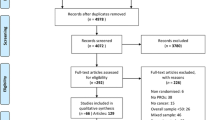
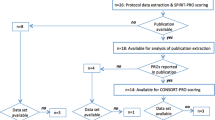
Published, phase III, ovarian cancer RCTs with PRO endpoints were identified following a systematic search of Medline and Cochrane databases (Jan 2000 to Feb 2016). Corresponding RCT protocols were downloaded (if published) or obtained by contacting authors. Two investigators independently assessed adherence of PRO-specific content of included protocols to a checklist of 58 recommended PRO protocol items currently being developed by the International Society for Quality of Life Research. Discrepancies were resolved with a third investigator.
Results
Of 41 eligible trials identified, 26 protocols were assessed (developed 1995–2010). We were unable to obtain the remaining 15 protocols. Protocols addressed a mean of 28 % PRO checklist items (range 8–66 %). Fifteen (58 % of assessed protocols) provided a rationale for PRO assessment, 8 (31 %) described a PRO objective, 24 (92 %) included a PRO assessment schedule, but only 6 (23 %) justified timing of PRO assessments. Twelve protocols (46 %) provided staff data collection instructions, 4 (15 %) included plans for monitoring PRO compliance, and 16 (62 %) included a PRO analysis plan.
Conclusions
On average, protocols addressed less than one-third of PRO protocol checklist items. In some cases, key guidance regarding PRO administration was lacking, which may lead to inconsistent and sub-optimal PRO methodology. Efforts are needed to improve PRO protocol content in cancer trials.


Similar content being viewed by others
References
King, M. T., Stockler, M. R., Butow, P., O’Connell, R., Voysey, M., Oza, A. M., et al. (2014). Development of the measure of ovarian symptoms and treatment concerns: Aiming for optimal measurement of patient-reported symptom benefit with chemotherapy for symptomatic ovarian cancer. International Journal of Gynecological Cancer, 24(5), 865–873.
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66(1), 7–30. doi:10.3322/caac.21332.
American Cancer Society (2013). Cancer facts & figures 2013. In American Cancer Society (Ed.). Atlanta.
Friedlander, M. L., & King, M. T. (2013). Patient-reported outcomes in ovarian cancer clinical trials. Annals of Oncology, 24(suppl 10), x64–x68.
Hess, L. M., & Stehman, F. B. (2012). State of the science in ovarian cancer quality of life research: A systematic review. International Journal of Gynecological Cancer, 22(7), 1273–1280. doi:10.1097/IGC.0b013e318263f02e.
Chan, A. W., Tetzlaff, J. M., Gotzsche, P. C., Altman, D. G., Mann, H., Berlin, J. A., et al. (2013). SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. British Medical Journal, 8(346), e7586.
Calvert, M., Kyte, D., von Hildebrand, M., King, M., & Moher, D. (2015). Putting patients at the heart of health-care research. The Lancet, 385(9973), 1073–1074.
International Committee of Medical Journal Editors (2016). Clinical Trial Registration. http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html. Accessed 6 April 2016.
Kyte, D., Duffy, H., Fletcher, B., Gheorghe, A., Mercieca-Bebber, R., King, M., et al. (2014). Systematic evaluation of the patient reported outcome (PRO) content of clinical trial protocols. PLoS One, 9(10), e110229.
Calvert, M., Kyte, D., Duffy, H., Gheorghe, A., Mercieca-Bebber, R., Ives, J., et al. (2014). Patient reported outcome (PRO) assessment in clinical trials: A systematic review of guidance for trial protocol writers. PLoS One, 9(10), e110216.
Chan, A.-W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013). SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine, 158(3), 200–207.
European Medicines Agency Oncology Working Party (2014). Draft Reflection Paper on the use of patient reported outcome (PRO) measures in oncology studies.
Food and Drug Administration (2009). Guidance for industry: Patient-reported outcome measures—Use in medical product development to support labeling claims.
Kyte, D., Reeve, B. B., Efficace, F., Haywood, K., Mercieca-Bebber, R., King, M. T., et al. (2016). International Society for Quality of Life Research commentary on the draft European Medicines Agency reflection paper on the use of patient-reported outcome (PRO) measures in oncology studies. Quality of Life Research, 25(2), 359–362.
Patrick, D. L., Guyatt, G. H., Acquadro, C., & Cochrane Patient Reported Outcomes Methods Group. (2011). Chapter 17: Patient-reported outcomes. In J. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions (Vol. 5.1.0.). London: The Cochrane Collaboration.
Aaronson, N. K. (1990). Quality of life research in cancer clinical trials: A need for common rules and language. Oncology, 4(5), 59–66.
Kyte, D., Ives, J., Draper, H., Keeley, T., & Calvert, M. (2013). Inconsistencies in quality of life data collection in clinical trials: A potential source of bias? Interviews with research nurses and trialists. PLoS One, 8(10), e76625. doi:10.1371/journal.pone.0076625.
Mercieca-Bebber, R., Palmer, M., Brundage, M., Calvert, M., Stockler, M., & King, M. (2015). Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: A systematic review. BMJO. doi:10.1136/bmjopen-2015-010938.
Kyte, D., Draper, H., & Calvert, M. (2013). Patient-reported outcome alerts: Ethical and logistical considerations in clinical trials. JAMA, 310(12), 1229–1230.
Kyte, D., Ives, J., Draper, H., & Calvert, M. (2016). Management of patient-reported outcome (PRO) alerts in clinical trials: A Cross sectional survey. PLoS One, 11(1), e0144658.
Skogvoll, E., & Kramer-Johansen, J. (2013). Publication of clinical trial protocols—what can we learn? Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 21, 12.
Weston, J., Dwan, K., Altman, D., Clarke, M., Gamble, C., Groves, T., et al. (2015). Selective reporting in clinical trials—An examination of discrepancy rates in pre-specified and reported outcomes in articles submitted to the BMJ. Trials, 16(2), 1. doi:10.1186/1745-6215-16-s2-o72.
Schandelmaier, S., Conen, K., von Elm, E., You, J. J., Blumle, A., Tomonaga, Y., et al. (2015). Planning and reporting of quality-of-life outcomes in cancer trials. Annals of Oncology, 26(9), 1966–1973.
Glasziou, P., Altman, D. G., Bossuyt, P., Boutron, I., Clarke, M., Julious, S., et al. (2014). Reducing waste from incomplete or unusable reports of biomedical research. The Lancet, 383(9913), 267–276.
Friedlander, M., Mercieca-Bebber, R., & King, M. (2016). Patient reported outcomes in ovarian cancer clinical trials—Lost opportunities and lessons learned. Annals of Oncology, 27(S1), i66–i71.
Acknowledgments
We are grateful to the Gynaecologic Cancer Inter-Group (GCIG) Symptom Benefit Working Group (SBWG), ImPROVeD Contributor’s Group leaders Professors Michael Friedlander, Felix Hilpert and Florence Joly, and to RCT investigators for providing access to trial protocols; specifically (in alphabetical order), D.K. Armstrong, J. Berek, N. Colombo, M. Bookman, M. Brady, J. Bryce, A. du Bois, G. Elser, A. Harkin, S. Kaye, J. Ledermann, K. Lindemann, B. Monk, T. Perren, M. Piccart, S. Pignata, J. Pfisterer, E. Pujade-Lauraine, N. Reed, G. Rustin, J. Sehouli, P. Vasey, and I. Vergote. We also acknowledge F. Roncolato and A. Long for their assistance identifying eligible trials and the International Society for Quality of Life Research Best Practices for PROs Protocol Checklist Taskforce for their contribution to developing the PRO Protocol Checklist.
Funding
This project did not receive specific grant funding. RMB is supported by Sydney Catalyst courtesy of the Cancer Institute NSW. MK is supported by the Australian Government courtesy of Cancer Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics
This article is an analysis of PRO-specific content of RCT protocols. It did not involve direct study of human participants, and therefore, human ethics approval was not required.
Informed consent
This article is an analysis of PRO-specific content of RCT protocols. It did not involve direct study of human participants, and therefore, informed consent was not required.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11136-017-1578-5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mercieca-Bebber, R., Friedlander, M., Kok, PS. et al. The patient-reported outcome content of international ovarian cancer randomised controlled trial protocols. Qual Life Res 25, 2457–2465 (2016). https://doi.org/10.1007/s11136-016-1339-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1339-x