Abstract
Background
To understand our performance with respect to the collection and reporting of patient-reported outcome (PRO) measure (PROM) data, we examined the protocol content, data completeness and publication of PROs from interventional trials conducted at the Royal Marsden NHS Foundation Trust (RM) and explored factors associated with data missingness and PRO publication.
Design
From local records, we identified closed, intervention trials sponsored by RM that opened after 1995 and collected PROMs as primary, secondary or exploratory outcomes. Protocol data were extracted by two researchers and scored against the SPIRIT-PRO (PRO protocol content checklist; score 0–100, higher scores indicate better completeness). For studies with locally held datasets, the information team summarized for each study, PRO completion defined as the number of expected (as per protocol) PRO measurements versus the number of actual (i.e. completed) PRO measurements captured in the study data set. Relevant publications were identified by searching three online databases and chief investigator request. Data were extracted and each publication scored against the CONSORT-PRO (PRO manuscript content checklist; scored as SPIRIT-PRO above). Descriptive statistics are presented with exploratory comparisons of point estimates and 95% confidence intervals.
Results
Twenty-six of 65 studies were included in the review. Nineteen studies had accessible datasets and 18 studies published at least one article. Fourteen studies published PRO results. Most studies had a clinical (rather than PRO) primary outcome (16/26). Across all studies, responses in respect of 35 of 69 PROMs were published. Trial protocols scored on average 46.7 (range 7.1–92.9) on the SPIRIT-PRO. Among studies with accessible data, half (10/19) had less than 25% missing measurements. Publications scored on average 80.9 (range 36–100%) on the CONSORT-PRO. Studies that published PRO results had somewhat fewer missing measurements (19% [7–32%] vs 60% [− 26 to 146%]). For individual PROMs within studies, missing measurements were lower for those that were published (17% [10–24%] vs 41% [18–63%]). Studies with higher SPIRIT-PRO scores and PROs as primary endpoints (13% [4–22%] vs 39% [10–58%]) had fewer missing measurements.
Conclusions
Missing data may affect publication of PROs. Extent of inclusion of SPIRIT-PRO protocol items and PROs as primary endpoints may improve data completeness. Preliminary evidence from the study suggests a future larger study examining the relationship between PRO completion and publication is warranted.
Similar content being viewed by others
Introduction
The importance of patient-reported outcomes (PROs) is increasingly recognised in cancer research [1]. PROs refer to “reports coming directly from patients about how they feel or function in relation to a health condition and its therapy without interpretation by healthcare professionals or anyone else” [2]. PROs may include concepts such as symptoms, quality of life, functional status or health and its treatment. Patient-reported outcome measures (PROMs) are tools for capturing PROs, usually in the form of questionnaires [3]. Using PROMs to investigate how medicinal and non-medicinal interventions effect patients’ lives beyond routine clinical outcomes in ways such as role functioning or social functioning provides richer information to enable patients, clinicians and commissioners to make appropriate clinical decisions.
Despite growing interest in PRO research, studies show there is room for improvement in the design, collection, analysis and reporting of PROs [4,5,6,7,8]. A recent review of trials that collected PRO data found a third did not include PRO-specific information in the protocol, with incomplete details in those that did [5]. Perhaps more disconcertingly, over a third of the studies in the review failed to publish the PRO results in a primary or secondary article. This raises an important ethical question, as data captured using these instruments but not reported is a waste of patient and researcher time and resource. To address deficits in the specification and reporting of PROs, PRO extension requirements have been added to the SPIRIT and CONSORT standards encouraging investigators to address critical requirements for protocol content and reporting when using PROs in clinical trials [9,10,11]. The checklists allow researchers to score protocols and publications to indicate the level of ‘completeness’ of PRO information.
One limitation in the Kyte et al. study was the lack of access to study datasets to explore whether the PRO data was ever initially captured. While there is recognition that missing data is a key issue in PRO research, it is unclear to what extent missing data contributes to failure to publish [12]. While funding and regulatory bodies are starting to encourage public availability of data, actual access to datasets is still lacking [13]. Even among trials that make information available, unpublished data is unlikely to be shared, limiting the ability to answer this question.
To have equal access to published and unpublished data, we explored the relationship between missing PRO data and publication by interrogating the study data sets available in the central trial database repository (CRS Web) at our institution. Specifically looking at interventional studies that planned to use PROs, our main objectives were to (1) describe our performance with respect to the completeness of PRO data collected and reported, (2) explore study-related factors associated with PRO data completeness and reporting and (3) examine the extent to which studies addressed SPIRIT-PRO and CONSORT-PRO requirements [9, 10].
Methods
Protocol registration
The protocol for this service evaluation was registered on Open Science Framework on 02/07/2020 (https://osf.io/ga3cn/).
Review process
The work was handled under the framework of a UK NHS Service Evaluation. This Service Evaluation (registration number: SE920) was approved by the Institution’s independent Committee for Clinical Research (CCR) which includes representatives from The Royal Marsden NHS Foundation Trust (RM) and Institute of Cancer Research (ICR). Access to encoded data to assess PRO completion was given only with Chief Investigator consent. Only the presence of a response was assessed. The responses themselves were not examined. PRO completion was summarised by the information team and exported for analysis to a trusted research environment with access limited to the Scoping Review Team. Access to withdrawal information was permitted in order to calculate the expected number of PRO measurements.
Study inclusion
Eligible studies included closed interventional studies sponsored by RM that intended to collect PROs. Using EDGE, the institution’s clinical research management system, we identified potential studies from the start of local digital records (1995). The search terms used to interrogate EDGE were ‘RM Sponsor’ AND ‘Interventional’ AND ‘Closed’. Interventions included clinical trials of investigative medicinal products (CTIMPs) and non-CTIMPs. Available trial protocols for all studies satisfying these criteria were provided by the Research and Development Department and screened for use of PROMs or patient reported experience measures (PREMs). Studies not intending to use PROMs or PREMs were excluded from the review. Included studies were assigned a random ID from 1 to 26.
Protocols: data extraction and SPIRIT-PRO scoring
Two reviewers (EL and HH) independently screened each protocol to extract relevant data using a pre-defined data extraction table (Additional file 1: Table S1) and scored each protocol using the SPIRIT-PRO checklist [10]. A third reviewer (LW) arbitrated discrepancies between reviewers. Data extracted included study characteristics, inclusion/exclusion criteria, funding source (commercial, academic or hybrid) and characteristics of each PROM used by each study. Healthcare cost measures do not fit entirely within our definition of PROMs, however, we decided to include these in our review as they add burden to participants and may be at particular risk for under-reporting as they require specific expertise for analysis.
In total, 25 data items were extracted per protocol with an additional 4 PROM specific items extracted for each PROM used. For each protocol, all 16 SPIRIT-PRO checklist items were recorded as ‘present’, ‘absent’ or ‘not applicable’. Percent agreement was calculated between reviewers as a measure of inter-rater reliability. A total SPIRIT-PRO score ranging from 0 to 100 was calculated for each study as the number of checklist items present divided by the sum of items present and absent (present/(present + absent)). This allowed us to adjust the denominator where checklist items were deemed ‘not applicable’. Summary tables were compiled for study characteristics, PROM characteristics and SPIRIT-PRO scores.
Trial data: acquisition and aggregation
For all included studies, permission from the Chief Investigator (CI) was sought to access anonymized trial data sets held on the Institution’s centralised trial database platform. Where trial data sets were not available from the central platform, the CI shared the PRO data directly with the information team.
On receipt of CI permission, the Information team summarized the completeness of the data for each PROM in each study to provide researchers with an aggregate dataset for analysis. This PROM-level information included the estimated number of expected and missing ‘measurements’, defined here as the unique completion of a PROM by each participant at each timepoint, and (among present measurements) the number of expected and missing items. Expected and missing measurements for each PROM were estimated by multiplying the number of timepoints expected (as detailed in the protocol schedule of assessments) by the number of participants in the study. The expected number was reduced with early withdrawal due death or study exit. Where available, study publications were examined to verify number of withdrawals. Expected items for each PROM were equivalent to the total number of items in the PROM multiplied by the number of available measurements. Missing items were the total number of missing items for the PROM in the data sets across all measurements. The number missing was then divided by the number expected to get percent missing for both measurements and items.
The Information team then imported the aggregate-level PRO completion data sets for each study into BRIDgE, the Institution’s trusted research environment. Access to BRIDgE was password protected and limited to the Scoping Review team, who combined the PRO completion data with protocol and publication data sets for analysis.
Publications: identification, data extraction and CONSORT-PRO scoring
To identify all available published articles, researchers agreed search terms for interrogation of online databases a-priori (available in the protocol). Literature searches were then undertaken independently by two researchers for each study using Medline, Embase and PsycINFO. Full articles of all trial-related primary and secondary publications were obtained. Where articles were not readily identifiable or available via online sources, the CI was contacted to determine whether an article had been published. All available publications were independently screened by two researchers and relevant data extracted using a pre-defined data extraction table (Additional file 2: Table S2). Data extracted included publication characteristics, sample demographics and PRO results. Discrepancies between reviewers were arbitrated by a third reviewer.
Articles that reported PRO results were then scored by two independent reviewers (EL and LW) using the CONSORT-PRO checklist [10]. For each article all 14 CONSORT-PRO checklist items were recorded as ‘present’, ‘absent’ or ‘not applicable’. The total score ranging from 0 to 100 was calculated using the same method as the SPIRIT-PRO above. Percent agreement between reviewers was calculated.
Statistical methods
The aggregate-level, PRO completion data for each study was linked with the protocol and publication data sets in BRIDgE and analysed using ‘R’ (version 4.04). Descriptive statistics were calculated using frequency and percentage for categorical data or mean and standard deviation for continuous data. Absent SPIRIT-PRO and CONSORT-PRO criteria are described. The review was not formally powered and due to relatively small number of studies included, statistical analysis was limited. However, we present selected exploratory comparisons with point estimates and 95% confidence intervals (CIs) using the t-distribution. Confidence intervals for proportions were calculated using Wilson intervals. We first analyzed the data at study-level and then, to further investigate variation in missingness and publication, we analyzed the data for each PROM across all studies, referred to as ‘PROM-level’ analysis.
Results
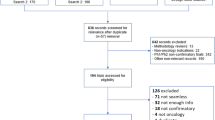
Interrogation of EDGE revealed 65 closed, interventional, RM-sponsored studies (Fig. 1). Of these, 26 (40.0%) studies planned to collect PRO data and were eligible for inclusion. Datasets were accessible for 19 studies and publications were identified for 18 studies, of which 14 published PRO results. In total, 11 studies had all information (i.e. protocol, publication and aggregate PRO completion data sets) available.
Protocols
Study characteristics: Studies included participants with a range of cancer types with the largest proportion (7/26) recruiting participants with cancers of mixed origin (Table 1). In ten studies (38.5%), the primary outcome was a PRO. Four of these were in the palliative care setting, two for treatment of lung cancer, one in the intensive care setting, one in complementary therapies, one examining hyperbaric therapy and one in gastrointestinal cancers. A wide range of interventions were evaluated ranging from device trials (n = 3), complimentary therapy (n = 3), anti-cancer systemic therapy (n = 4) and dietary interventions (n = 2). The majority (19/26) were randomised controlled trials by design, academically funded (19/26) and single-centre studies (17/26). The median (range) year of trial start was 2010 (2000–2015) and closure was 2014 (2011–2018). The average number of studies using PROs increased over time from 1.2 studies per year between 2000 and 2007 to 2.7 studies per year between 2008 and 2015. However, the proportion of studies using PROs decreased over the same time periods from 34 to 23%. Median planned sample size was 84 (25–730). The combined target recruitment for the 26 included studies amounted to 3157 participants.
PRO characteristics: A total of 60 PROMs (49 unique instruments) were used across the 26 studies with a combined total of 154 time points resulting in a planned 41,736 PROM measurements (Table 2). The most commonly used measure was the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Core Module (QLQ-C30) (10 trials; 39%). Where cancer site-specific EORTC modules were used, these were always used in conjunction with the QLQ-C30 (as recommended). The vast majority of PROMs used (36/49; 74%) were validated. Non-validated questionnaires measured healthcare costs, tolerability, toxicity, sleep and patient experience. The median number of PROMs employed was 2 and ranged from 1 (7/26 trials) to 8 (1/26 trials) with the majority of trials (10/26) using 2 PROMs (Table 3). The most commonly measured PRO concept was quality of life (58% of trials) followed by symptoms (35%) and patient experience (31%). Other concepts reported included anxiety / depression, health status, economics, well-being and physical function (Table 3).
SPIRIT-PRO scores Trial protocols scored an average of 46.7 (range 7.1–92.9) on the SPIRIT-PRO checklist (Fig. 2). Reviewers achieved 81% agreement. Protocols included an average of 7 (range 1–13) of the 16 checklist items (Fig. 2). SPIRIT-PRO recommendations most frequently absent included item 10 (availability of multiple language versions of PROMs) and 16 (real-time monitoring of PRO responses) each omitted in 25 protocols; item 1 (responsibility for PRO content of protocol) absent in 21 protocols and item 15 (handling of missing data) absent in 19 protocols. Items 11 (inclusion of proxy reported outcomes) and 4 (PRO specific eligibility criteria) were deemed ‘not applicable’ in 25 and 15 protocols respectively. Trials obtaining the five highest SPIRIT-PRO scores were for trials evaluating supportive care (n = 1), complementary therapy (n = 1), dietary (n = 1), bariatric therapy (n = 1) and systemic treatment (n = 1) interventions.
Trial data
Aggregate PRO completion data sets were available for 73% (19/26) of eligible studies. At the study-level, 53% (10/19) of studies had less than 25% missing measurements, 37% (7/19) of studies had between 26 and 50% missing measurements and 11% (2/19) of studies had more than 50% missing measurements. Among the 19 studies with data available, 24,402 measurements were collected of the 33,006 planned (74%). Data were accessible for three of the four studies that published a paper without publishing PRO results. Missing measurements for these studies ranged from 39 to 100%.
At the PROM-level, the studies planned to collect 69 PROMs. Data were available for 54 PROMs across the 19 studies with accessible data sets. Sixty-three percent of PROMs (34/54) had 25% or fewer missing measurements, 22% of PROMs (12/54) had between 26 and 50% missing measurements and 15% of PROMs (8/54) had greater than 50% missing measurements. Among available measurements with more than one item (n = 46), 83% of PROMs (45/46) had 25% or fewer missing items and only one PROM (1/46) had between 26 and 50% missing items.
Publications
Article characteristics Publications were available for 18 (69%) of the 26 eligible studies, of which 78% (14/18) published results for at least one PRO. Overall, of the 26 studies intending to collect PRO data using 69 different PROMS, fourteen studies collectively published 35 (51%) of the intended PROMs. Of those that published PROs, the median time between study closure and publication of PRO results was 2 years (range − 1 to 3). Median year of closure for studies without an identified publication (8/26) was 2016 (range 2011–2018). Information about the final sample size was found in either the data or publication for 23 studies and indicated 61% (14/23) of studies achieved the planned sample size.
CONSORT-PRO scores Publications containing PRO results were available for 14 studies. Where more than one publication reported PRO results for a study, the primary publication was scored. An average of 9 (range 4–13) of the 14 CONSORT-PRO checklist items were present in each publication. Overall, publications scored an average of 80.9 (range 36.4–100.0) (Fig. 2). Reviewers achieved 77% agreement. CONSORT-PRO recommendations most frequently absent included item 7 (approaches for dealing with missing data), which was absent in 7 publications, item 9 (baseline PRO data) absent in 5 publications and item 3 (PRO hypothesis) absent in 5 publications. Items 4 (PRO specific eligibility criteria) and 12 (additional PRO analyses) were deemed ‘not applicable’ in 12 and 10 publications respectively. Trials obtaining the five highest CONSORT-PRO scores evaluated supportive care (n = 1), complementary therapy (n = 2) and dietary (n = 2) interventions.
Study-level analysis
Compared to studies with a primary clinical endpoint (16/26), studies with a primary PRO endpoint had higher mean [95% CI] SPIRIT-PRO scores (35.2 [23.2–47.3] vs 65.1 [51.3–78.9], respectively). Looking at studies that published at least one manuscript, there was no difference in the percentage of studies [95% CI] publishing PRO results between studies with a primary PRO endpoint (7/18) and those with a clinical endpoint (86% [49–97%] vs. 73%, [43–90%], respectively). Mean [95% CI] SPIRIT-PRO scores did not differ between studies that published at least one PRO (14/18) and those that did not (44.5 [28.9–60.1] vs 32.1 [− 4.3 to 68.6], respectively).
However, among studies that published PRO results, studies with primary PRO endpoints (6/14) had slightly higher mean [95% CI] CONSORT-PRO scores (90.6 [77.9–103.3] vs 73.4 [58.5–88.3] in studies with clinical endpoints). A SPIRIT-PRO score of > 50.0 appears to consistently result in higher CONSORT-PRO scores (Fig. 2).
Focusing on studies with PRO completion data sets available, those with primary PRO endpoints (9/19) had lower mean [95% CI] percentage of missing measurements (13% [4–22%] vs 39% [20–57%] in studies with primary clinical endpoints). There was no difference in mean [95% CI] percentage of missing measurements between single centre (12/19) and multi-centre studies (21% [9–32%] vs 36% [7–65%], respectively). Studies with higher SPIRIT-PRO scores were likely to have fewer missing measurements as SPIRIT-PRO scores and the percent of missing measurements at the study-level were negatively correlated (n = 19; r = − 0.53). Among studies with data that published at least one manuscript and included at least one PRO (11/14), the mean [95% CI] percentage of missing measurements was somewhat lower compared to studies that published a manuscript but did not include any PRO results (19% [7–32%] vs 60% [− 26 to 146%], respectively).
PROM-level analysis
Studies that published at least one manuscript accounted for 51 of the 69 (74%) PROMs intended to be collected. Among studies that published, the percentage [95% CI] of PROMs used for primary outcomes (7/51) that were published was no different than those used for secondary outcomes (86% [49–97%] vs 66% [51–78%], respectively).
Summary missingness information was available for 54 PROMs. Among studies with summary information, PROMs used for primary objectives (9/54) had lower mean [95% interval] percentage of missing measurements than PROMs used for secondary objectives (7% [4–10%] vs 26% [18–33%], respectively). The mean [95% CI] percentage of missing measurements was lower among PROMs with published results (30/41) compared to non-published PROMs (17% [10–24%] vs 41% [18–63%], respectively).
Discussion
This service evaluation sought to explore our Institution’s performance with respect to the completeness of PRO data collected and reported in intervention studies and identify research study specific factors associated PRO data missingness. Nearly half the studies included in our evaluation planned to capture PRO data reflecting the importance investigators attach to these measures. However, one third of trials have yet to publish any results 3–10 years since trial closure. Among those that published at least one paper, nearly a quarter did not include any PRO results and almost a third of questionnaires were never published.
We found the omission of PRO results in publications may be influenced by the degree of missing data. In this evaluation, the mean percentage of missing measurements was higher for PROMs that were not published compared to those that were published. A similar pattern to some extent was found at study-level. Furthermore, the three studies that published at least one paper without PRO results for which we had data all had more than 50% missing measurements. While our sample size is small, the findings suggest the extent of PRO data missingness may be a factor in non-publication.
We hypothesize that the completeness of PRO data may be related to the importance given to the PRO data in trial set up and conduct. Indeed, Kyte et al. found that protocols were more likely to be complete and results published where the primary outcome was a PRO [5]. Our results on data missingness align with these findings as the percentage of missing PRO measurements was lower among trials with a primary PRO endpoint compared to those with a primary clinical endpoint. This may reflect trial staff perception that primary endpoint data is more important than secondary endpoint data. Similarly, at PROM-level, we found that for PROMs employed as primary endpoints, the mean number of missing measurements was lower than for PROMs used for secondary objectives. To improve PRO data completeness by increasing perceived importance, investigators could include PROs as co-primary endpoints or use behaviour theory-informed motivational information in the protocol and trial staff training.
Our analysis of the 19 studies for which aggregate PRO completion data sets were available revealed that about half the trials had more than 25% missing measurements including two trials which had over 50% missingness. The reason for missing PRO data could reflect either that the required PRO data were not captured at all or that the data were captured and not transcribed into an accessible digital platform. Few trial protocols included in this review outlined the mode of PRO capture. Most will have used paper questionnaires, which can be cumbersome, resource-intensive and potentially prone to transcription error. Electronic capture of PRO data directly from patients shows promise but is yet to be tested in nested randomized trials. RM has recently implemented an electronic questionnaire system developed in the Netherlands called PROFILES [14, 15]. We hope to evaluate its effect on our performance in respect of PRO data capture and reporting in the future and are satisfied that we now have a reliable benchmark to assess our future performance and encourage improvements.
One way of improving the publication of PRO results is to improve the PRO-related content of trial protocols using the SPIRIT-PRO checklist. Previous research suggests that including SPIRIT-PRO checklist items improves the completeness of PRO information in protocols and may reduce the risk of bias [16]. Although publication of the SPIRIT-PRO extension in July 2018 post-dates the protocols included in this analysis, it is encouraging to see that adherence to the guidelines compares well with previously published results [5]. Our own analysis supports the hypothesis that higher SPIRIT-PRO scores have beneficial effects. In the 19 studies for which data sets were available for analysis, we found that higher SPIRIT-PRO scores were associated with fewer missing measurements. Investigators in future should make use of the SPIRIT-PRO in the protocol-writing phase. Based on the results of this evaluation, RM has implemented standard review of protocols against the SPIRIT-PRO as part of the sponsorship process to improve PRO data collection.
SPIRIT-PRO scores were higher in studies with primary PRO endpoints compared to those with primary clinical endpoints suggesting that a more thorough approach to protocol development is adopted when PROs are the primary endpoints. Similarly, CONSORT-PRO scores were higher amongst studies using PROs as the primary endpoint compared to those with primary clinical endpoints, which suggests rigor in planning translates into rigorous reporting. Adherence of the published articles to CONSORT-PRO criteria in our cohort was substantially higher than the results published by Kyte et al. [5].
This study is limited by the small sample size and single institution nature so all findings should be considered exploratory. Some of the very large confidence intervals reflect that the true estimate could not be determined based on the small sample. However, this preliminary evidence of a relationship between missing PRO data and publication provides rationale for a future research initiative to explore the relationship in more depth. The findings also suggest important potential areas for improvement in PRO research, including the need to include comprehensive PRO information in study protocols and prioritise the collection of PROs to encourage complete data and publication of results. Future research could also look in more depth at how missingness relates to specific PROMs and used to guide selection of instruments in trial design.
Conclusion
Missing data may impact the publication of PROs. The completeness of SPIRIT-PRO checklist items in the study protocol and the use of PROs as primary endpoints may improve data completeness. This preliminary evidence suggests a future larger review investigating the relationship between PRO data missingness and publication is warranted.
Availability of data and materials
The dataset analysed during the current study are held in the Royal Marsden Data Warehouse and are available from the corresponding author on reasonable request.
Abbreviations
- PRO:
-
Patient-reported outcome
- PROM:
-
Patient reported outcome measure
- CTIMP:
-
Clinical trials of investigative medicinal product
- PREM:
-
Patient-reported experience measure
- CI:
-
Chief investigator
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- QLQ-C30:
-
Quality of Life Questionnaire-Core Module
References
Weldring T, Smith SMS (2013) Patient-reported outcomes (pros) and patient-reported outcome measures (PROMS). Health Serv Insights 6:61–68. https://doi.org/10.4137/HSI.S11093
Collaboration TC (2008) Cochrane handbook for systematic reviews of interventions: cochrane book series, 5.1.0. Wiley, Hoboken
Kluetz PG, Slagle A, Papadopoulos EJ et al (2016) Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-15-2035
Kyte D, Duffy H, Fletcher B et al (2014) Systematic evaluation of the patient-reported outcome (PRO) content of clinical trial protocols. PLoS ONE 9:e110229. https://doi.org/10.1371/journal.pone.0110229
Kyte D, Retzer A, Ahmed K et al (2019) Systematic evaluation of patient-reported outcome protocol content and reporting in cancer trials. JNCI: J Natl Cancer Inst 111:1–9. https://doi.org/10.1093/jnci/djz038
Brundage M, Bass B, Davidson J et al (2011) Patterns of reporting health-related quality of life outcomes in randomized clinical trials: implications for clinicians and quality of life researchers. Qual Life Res 20:653–664. https://doi.org/10.1007/s11136-010-9793-3
Kyte D, Ives J, Draper H et al (2013) Inconsistencies in quality of life data collection in clinical trials: a potential source of bias? Interviews with research nurses and trialists. PLoS ONE 8:1–11. https://doi.org/10.1371/journal.pone.0076625
Efficace F, Fayers P, Pusic A et al (2015) Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient-reported outcome extension: a pooled analysis of 557 trials. Cancer 121:3335–3342. https://doi.org/10.1002/cncr.29489
Calvert M, Blazeby J, Altman DG et al (2013) Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA: J Am Med Assoc 309:814–822. https://doi.org/10.1001/jama.2013.879
Calvert M, Kyte D, Mercieca-Bebber R et al (2018) Guidelines for inclusion of patient-reported outcomes in clinical trial protocols the spirit-pro extension. JAMA: J Am Med Assoc 319:483–494. https://doi.org/10.1001/jama.2017.21903
Bottomley A, Pe M, Sloan J et al (2016) Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol 17:e510–e514. https://doi.org/10.1016/S1470-2045(16)30510-1
Mercieca-Bebber R, Palmer MJ, Brundage M et al (2016) Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open 6:e010938. https://doi.org/10.1136/bmjopen-2015-010938
Hopkins AM, Rowland A, Sorich MJ (2018) Data sharing from pharmaceutical industry sponsored clinical studies: audit of data availability. BMC Med 16:1–6. https://doi.org/10.1186/s12916-018-1154-z
Meirte J, Hellemans N, Anthonissen M et al (2020) Benefits and disadvantages of electronic patient-reported outcome measures: systematic review. JMIR Perioper Med 3:e15588. https://doi.org/10.2196/15588
Van De Poll-Franse LV, Horevoorts N, Van EM et al (2011) The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 47:2188–2194. https://doi.org/10.1016/j.ejca.2011.04.034
Rivera SC, Kyte DG, Aiyegbusi OL et al (2019) The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis. Health Qual Life Outcomes 17:1–19. https://doi.org/10.1186/s12955-019-1220-z
Acknowledgements
We would like to thank the information team for all the support summarising and aggregating PRO completion information for each study and advising on the use of the digital research environment. We would also like to thank all the chief investigators for allowing the information team to summarise PRO completion information from their studies.
Funding
This study represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funder was not involved in the study design, data collection, analysis and interpretation or manuscript writing.
Author information
Authors and Affiliations
Contributions
EL, LW, EY, OH and HC conceptualization and methodology; EL LW, AG, SM and HH investigation; AG, JL, LS, RT resources; SM data curation; EL and LW formal analysis; AG, JL, OH, RT, CP, SC and KM supervision; LW and EL writing—original draft; EL, HH, AG, JL, EY, HC, CP, KM, SM, LS, OH, SC, RT and LW writing—review and editing; EL and LW data visualisation; EL, HH and LW project administration; LS and RT funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This review was approved by the Royal Marsden Committee for Clinical Research as a Service Evaluation (SE920). The chief investigator of each study gave consent to access the data. The review protocol was registered on the Open Science Framework in July 2020 and review activities commenced in the same month (https://osf.io/registries/).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Protocol data extraction table.
Additional file 2: Table S2
. Publication data extraction table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lidington, E., Hogan, H., Gandolfi, A. et al. Assessing the collection and reporting of patient-reported outcome data in interventional cancer trials: a single institution, retrospective systematic evaluation. J Patient Rep Outcomes 6, 128 (2022). https://doi.org/10.1186/s41687-022-00529-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41687-022-00529-9