Abstract
Purpose
To develop a mapping algorithm for a conversion of the EORTC QLQ-C30 and EORTC QLQ BR-23 into the EQ-5D-derived utilities in metastatic breast cancer (MBC) patients.
Methods
We enrolled 199 patients with MBC from four leading Korean hospitals in 2009. EQ-5D utility, cancer-specific (QLQ-C30) and breast cancer-specific quality of life data (QLQ-BR23) and selected clinical and demographic information were collected from the study participants. Ordinary least squares regression models were used to model the EQ-5D using QLQ-C30 and QLQ-BR23 scale scores. To select the best model specification, six different sets of explanatory variables were compared.
Result
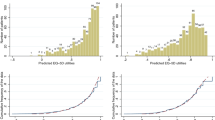
Regression analysis with the multiitem scale scores of QLQ-C30 was the best-performing model, explaining for 48.7% of the observed EQ-5D variation. Its mean absolute error between the observed and predicted EQ-5D utilities (0.092) and relative prediction error (2.784%) was among the smallest. Also, this mapping model showed the least systematic errors according to disease severity.
Conclusions
The mapping algorithms developed have good predictive validity, and therefore, they enable researchers to translate cancer-specific health-related quality of life measures to the preference-adjusted health status of MBC patients.
Similar content being viewed by others
Abbreviations
- CUA:
-
Cost-utility analysis.
- ECOG:
-
Eastern cooperative oncology group
- EORTC QLQ-BR23 (or QLQ-BR23):
-
European Organization for Research and Treatment of Cancer quality of life questionnaire breast cancer-23
- EORTC QLQ-C30 (or QLQ-C 30):
-
European Organization for Research and Treatment of Cancer quality of life questionnaire core-30
- EQ-VAS:
-
EQ-5D visual analogue scale
- ISPOR:
-
International society for pharmacoeconomics and outcomes research
- MBC:
-
Metastatic breast cancer
- NHI:
-
National health insurance
- OLS:
-
Ordinary least squares
- QALYs:
-
Quality-adjusted life years
- QOL:
-
Quality of life
- RPE:
-
Relative prediction error
- VIF:
-
Variance inflation factor
References
Kontodimopoulos, N., Aletras, V. H., Paliouras, D., & Niakas, D. (2009). Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D, and 15 D instruments. Value in Health, 12(8), 1151–1157.
Patrick, D. L., & Deyo, R. A. (1989). Generic and disease-specific measures in assessing health status and quality of life. Medical Care, 27(Supp l), S217–S232.
Rivero-Arias, O., Quellet, M., Gray, A., Wolstenhome, J., Rothwell, P. M., & Luengo-Fernandez, R. (2009). Mapping the modified rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Medical Decision Making, 30(3), 341–354.
Cheung, Y. B., Tan, L. C. S., Lau, P. N., Au, W. L., & Luo, N. (2008). Mapping the eight-item Parkinson’s Disease Questionnaire (PDQ-8) to the EQ-5D utility index. Quality of Life Research, 17, 1173–1181.
Bottomley, A., & Aaronson, N. K. (2007). International perspective on health-related quality-of-life research in cancer clinical trials: The European Organization for Research and Treatment of Cancer experience. Journal of Clinical Oncology, 25(32), 5082–5086.
Aaronson, N. K., Ahmedzai, S., Bergman, B., et al. (1993). The European organization for research and treatment of cancer QLQ C-30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85(6), 365–376.
McKenzie, L., & Pol, M. (2009). Mapping the EORTC QLQ C-30 onto the EQ-5D instrument: The potential to estimate QALYs without generic preference data. Value in Health, 12(1), 167–171.
Wu, E. Q., Mulani, P., Farrell, M. H., & Sleep, D. (2007). Mapping FACT-P and EORTC QLQ-C30 to patient health status measured by EQ-5D in metastatic hormone-refractory prostate cancer patients. Value in Health, 10(5), 408–414.
Crott, R., & Briggs, A. (2010). Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. The European Journal of Health Economics, 11(4), 427–434.
Korea National Cancer Information Center. (2010). Prevalence of cancer. cited August 28, 2010, from URL http://www.cancer.go.kr/cms/statistics/stat/1373001_1611.html.
Health Insurance Review and Assessment Services. (2006). Korean pharmaceutical economic evaluation guidelines. Cited December 25, 2010, from URL http://www.ispor.org/PEguidelines/source/Korean_PE_Guidelines_Korean_Version.pdf.
Fayers, P. M., Aaronson, N. K., Bjordal, K., Curan, D., & Groenvold, M. (1999). On behalf of EORTC quality of life study group. EORTC QLQ-C30 scoring manual (2nd ed.). Brussels: EORTC.
Sprangers, M. A. G., Groenvold, M., Arraras, J. I., et al. (1996). the EORTC breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. Journal of Clinical Oncology, 14(10), 2756–2768.
The EuroQol Group. EQ-5D. Cited December 25, 2010, from URL http://www.euroqol.org.
Lee, Y. K., Nam, H. S., Chuang, L. H., et al. (2009). South Korean time trade-off values for EQ-5D health states: Modeling with observed values for 101 health states. Value in Health, 12(8), 1187–1193.
Borras, J. M., Sanchez-Hernandez, A., Navarro, M., et al. (2001). Compliance, satisfaction, and quality of life of patients with colorectal cancer receiving home chemotherapy or outpatient treatment: A randomized controlled trial. British Medical Journal, 322, 1–5.
World Health Organization. (1980). WHO handbook for reporting results of cancer treatment. Neoplasma, 20, 37–46.
Dakin, H., Petrou, S., Haggard, M., Benge, S., & Williamson, I. (2010). Mapping analyses to estimate health utilities based on responses to the OM8–30 otitis media questionnaire. Quality of Life Research, 19(1), 65–80.
Versteegh, M. M., Rowen, D., Brazier, J. B., & Stolk, E. A. (2010). Mapping onto EQ-5 D for patients in poor health. Health and Quality of Life Outcomes, 8, 141.
Brazier, J. E., Yang, Y., Tsuchiya, A., & Rowen, D. L. (2009). A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. European Journal of Health Economics, 2010(11), 215–225.
Rowen, D., Brazier, J., & Roberts, J. (2009). Mapping SF-36 onto the EQ-5 D index: How reliable is the relationship? Health and Quality of Life Outcomes, 7, 27.
Sauerland, S., Weiner, S., Dolezalova, K., Angrisani, L., Noguera, C. M., Garcia-Caballero, M., et al. (2009). Mapping utility scores from a disease-specific quality-of-life measure in Bariatric surgery patients. Value in Health, 12(2), 364–370.
Vittinghoff, E., Shiboski, S. C., Glidden, D. V., McCulloch, C. E. (2005). Regression methods in biostatistics: Linear, logistic, survival, and repeated measures models (Chap. 5, pp. 147–148). New York: Springer Science+Business Media, Inc.
SAS/STAT(R) 9.2 (2009). User’s Guide (2nd ed.), (Chap. 4, pp. 98). Cary, North Carolina, USA: SAS Institute Inc.
Korea Center for Disease Control and Prevention. (2007). The Korea national health and nutrition examination survey (KNHANES IV). Seoul: Korea Ministry of Health, Welfare, and Family Affairs, p. 290.
Acknowledgments
This study was supported by an unrestricted grant from Pfizer Pharmaceuticals Korea Limited.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Technical summary: principles of scoring of QLQ-C30
This was taken from the EORTC QLQ-C30 Scoring Manual [12].
-
1.
Calculate raw score
If items I 1, I 2,…I n are included in a scale, then the raw score of a scale is calculated as follows:
-
2.
Linear transformation
Apply the linear transformation to 0–100 to obtain the score S,
Functional scales: score = {1−(RS−1)/range} × 100
Symptom scales/items: score = {(RS−1)/range} × 100
Global health status/QOL: score = {(RS−1)/range} × 100
Range is the difference between the maximum possible value of RS and the minimum possible value. The QLQ-C30 has been designed so that all the items in any scale take the same range of values. Therefore, the range of RS equals the range of the item values. Most items are scored 1–4, giving a range = 3. The exceptions are the items contributing to the global health status/QOL, which are 7-point questions with a range = 6, and the initial yes/no item on the earlier versions of the QLQ-C30, which have range = 1.
Appendix 2
See Table 6.
Rights and permissions
About this article
Cite this article
Kim, Ej., Ko, SK. & Kang, HY. Mapping the cancer-specific EORTC QLQ-C30 and EORTC QLQ-BR23 to the generic EQ-5D in metastatic breast cancer patients. Qual Life Res 21, 1193–1203 (2012). https://doi.org/10.1007/s11136-011-0037-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-011-0037-y