Abstract
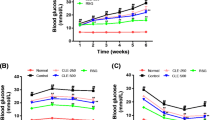
Lupinus albus seeds contain conglutin gamma (Cγ) protein, which exerts a hypoglycemic effect and positively modifies proteins involved in glucose homeostasis. Cγ could potentially be used to manage patients with impaired glucose metabolism, but there remains a need to evaluate its effects on hepatic glucose production. The present study aimed to analyze G6pc, Fbp1, and Pck1 gene expressions in two experimental animal models of impaired glucose metabolism. We also evaluated hepatic and renal tissue integrity following Cγ treatment. To generate an insulin resistance model, male Wistar rats were provided 30% sucrose solution ad libitum for 20 weeks. To generate a type 2 diabetes model (STZ), five-day-old rats were intraperitoneally injected with streptozotocin (150 mg/kg). Each animal model was randomized into three subgroups that received the following oral treatments daily for one week: 0.9% w/v NaCl (vehicle; IR-Ctrl and STZ-Ctrl); metformin 300 mg/kg (IR-Met and STZ-Met); and Cγ 150 mg/kg (IR-Cγ and STZ-Cγ). Biochemical parameters were assessed pre- and post-treatment using colorimetric or enzymatic methods. We also performed histological analysis of hepatic and renal tissue. G6pc, Fbp1, and Pck1 gene expressions were quantified using real-time PCR. No histological changes were observed in any group. Post-treatment G6pc gene expression was decreased in the IR-Cγ and STZ-Cγ groups. Post-treatment Fbp1 and Pck1 gene expressions were reduced in the IR-Cγ group but increased in STZ-Cγ animals. Overall, these findings suggest that Cγ is involved in reducing hepatic glucose production, mainly through G6pc inhibition in impaired glucose metabolism disorders.


Similar content being viewed by others
Abbreviations
- Cγ:
-
Gamma conglutin
- Fbp1 :
-
Fructose-bisphosphatase 1 gene
- G6pc :
-
Glucose-6-phosphatase gene
- IR:
-
Insulin resistance
- Pck1 :
-
Phosphoenolpyruvate carboxykinase 1 gene
- STZ:
-
Streptozotocin
- T2D:
-
Type 2 diabetes
References
Duranti M, Consonni A, Magni C, Sessa F, Scarafoni A (2008) The major proteins of lupin seed: characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci Technol 19:624–633
Lucas MM, Stoddard FL, Annicchiarico P, Frías J, Martínez-Villaluenga C, Sussmann D, Duranti M, Seger A, Zander PM, Pueyo JJ (2015) The future of lupin as a protein crop in Europe. Front Plant Sci 6:705. doi:10.3389/fpls.2015.00705
Kohajdová Z, Karovičová J, Schmidt Š (2011) Lupin composition and possible use in bakery – a review. Czech J Food Sci 29(3):203–211
Belski R, Mori TA, Puddey IB, Sipsas S, Woodman RJ, Ackland TR, Beilin LJ, Dove ER, Carlyon NB, Jayaseena V, Hodgson JM (2011) Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: a 12-month randomized controlled weight loss trial. Int J Obes 35(6):810–819
Bähr M, Fechner A, Krämer J, Kiehntopf M, Jahreis G (2013) Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: a randomized, controlled crossover study. Nutr J 12:107. doi:10.1186/1475-2891-12-107
Fontanari GG, Batistuti JP, da Cruz RJ, Saldiva PHN, Areas JAG (2012) Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate. Food Chem 132:1521–1526
Radtke J, Schutkowski A, Brandsch C, Hirche F, Hasenkopf K, Stangl GI (2015) Isolated conglutin γ from lupin, but not phytate, lowers serum cholesterol without influencing vascular lesion development in the ApoE-deficient mouse model. Plant Foods Hum Nutr 70(2):113–118
Brandsch C, Kappis D, Weisse K, Stangl GI (2010) Effects of untreated and thermally treated lupin protein on plasma and liver lipids of rats fed a hypercholesterolemic high fat or high carbohydrate diet. Plant Foods Hum Nutr 65(4):410–416
Magni C, Sessa F, Accardo E, Vanoni M, Morazzoni P, Scarafoni A, Duranti M (2004) Conglutin gamma, a lupin seed protein, binds insulin in vitro and reduces plasma glucose levels of hyperglycemic rats. J Nutr Biochem 15(11):646–650
Bertoglio JC, Calvo MA, Hancke JL, Burgos RA, Riva A, Morazzoni P, Ponzone C, Magni C, Duranti M (2011) Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 82(7):933–938
Terruzzi I, Senesi P, Magni C, Montesano A, Scarafoni A, Luzi L, Duranti M (2011) Insulin-mimetic action of conglutin-γ, a lupin seed protein, in mouse myoblasts. Nutr Metab Cardiovasc Dis 21(3):197–205
Lovati MR, Manzoni C, Castiglioni S, Parolari A, Magni C, Duranti M (2012) Lupin seed γ-conglutin lowers blood glucose in hyperglycaemic rats and increases glucose consumption of HepG2 cells. Br J Nutr 107(1):67–73
Vargas-Guerrero B, García-López PM, Martínez-Ayala AL, Domínguez-Rosales JA, Gurrola-Díaz CM (2014) Administration of Lupinus albus gamma conglutin (Cγ) to n5 STZ rats augmented ins-1 gene expression and pancreatic insulin content. Plant Foods Hum Nutr 69(3):241–247
Martínez-Ayala AL, Paredes-López O (2001) Molecular characterization of the β-conglutin of lupin seeds. J Food Biochem 25(1):15–31
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166(2):368–379
Car BD, Eng VM, Everds NE et al (2006) Clinical pathology of the rat. In: Suckow MA, Weisbroth SH, Franklin CA (eds) The laboratory rat, 2nd edn. Academic Press, San Diego, p 133
Takada J, Machado MA, Peres SB, Brito LC, Borges-Silva CN, Costa CE, Fonseca-Alaniz MH, Andreotti S, Lima FB (2007) Neonatal streptozotocin-induced diabetes mellitus: a model of insulin resistance associated with loss of adipose mass. Metabolism 56(7):977–984
Matteucci E, Giampietro O (2008) Proposal open for discussion: defining agreed diagnostic procedures in experimental diabetes research. J Ethnopharmacol 115(2):163–172
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Guo X, Li H, Xu H, Woo S, Dong H, Lu F, Lange AJ, Wu C (2012) Glycolysis in the control of blood glucose homeostasis. Acta Pharm Sin B 2(4):358–367
Wu C, Okar DA, Newgard CB, Lange AJ (2002) Increasing fructose 2,6-bisphosphate overcomes hepatic insulin resistance of type 2 diabetes. Am J Physiol Endocrinol Metab 282(1):E38–E45
Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC (1999) Within- and between-subject variation in commonly measured anthropometric and biochemical variables. Clin Chem 45(4):561–566
Tripathy D, Chavez AO (2010) Defects in insulin secretion and action in the pathogenesis of type 2 diabetes mellitus. Curr Diab Rep 10(3):184–191
Chitturi S, George J (2002) Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis 22(2):169–183
Choudhury D, Ahmed Z (2006) Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol 2(2):80–91
Van Schaftingen E, Gerin I (2002) The glucose-6-phosphatase system. Biochem J 362:513–532
Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57(2):306–314
Croniger CM, Chakravarty K, Olswang Y, Cassuto H, Reshef L, Hanson RW (2002) Phosphoenolpyruvate carboxykinase revisited: II. Control of PEPCK-C gene expression. Biochem Mol Biol Educ 30(6):353–362
Barthel A, Schmoll D (2003) Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 285(4):E685–E692
van Poelje PD, Potter SC, Erion MD (2011) Fructose-1, 6-bisphosphatase inhibitors for reducing excessive endogenous glucose production in type 2 diabetes. Handb Exp Pharmacol 203:279–301
Acknowledgements
The authors thank Ing. Rogelio Troyo-Sanromán for statistical advice and Dr. van Santen for the lupin seeds. This study was supported by a CONACyT grant (number 60283) given to CMGD. AEGS and BVG received fellowships from CONACyT (fellowship numbers 204929 and 225001, respectively). Also, partial financial support was received from University of Guadalajara REC/747/2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no potential conflicts of interest or financial disclosures.
Additional information
Ana E. González- Santiago and Belinda Vargas-Guerrero contributed equally to this work.
Rights and permissions
About this article
Cite this article
González-Santiago, A.E., Vargas-Guerrero, B., García-López, P.M. et al. Lupinus albus Conglutin Gamma Modifies the Gene Expressions of Enzymes Involved in Glucose Hepatic Production In Vivo . Plant Foods Hum Nutr 72, 134–140 (2017). https://doi.org/10.1007/s11130-016-0597-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-016-0597-7