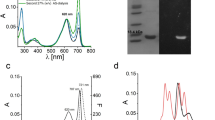
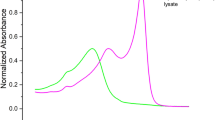
Abstract
Phycobilisomes (PBS), the major light-harvesting antenna in cyanobacteria, are supramolecular complexes of colorless linkers and heterodimeric, pigment-binding phycobiliproteins. Phycocyanin and phycoerythrin commonly comprise peripheral rods, and a multi-cylindrical core is principally assembled from allophycocyanin (AP). Each AP subunit binds one phycocyanobilin (PCB) chromophore, a linear tetrapyrrole that predominantly absorbs in the orange-red region of the visible spectrum (600–700 nm). AP facilitates excitation energy transfer from PBS peripheral rods or from directly absorbed red light to accessory chlorophylls in the photosystems. Paralogous forms of AP that bind PCB and are capable of absorbing far-red light (FRL; 700–800 nm) have recently been identified in organisms performing two types of photoacclimation: FRL photoacclimation (FaRLiP) and low-light photoacclimation (LoLiP). The FRL-absorbing AP (FRL-AP) from the thermophilic LoLiP strain Synechococcus sp. A1463 was chosen as a platform for site-specific mutagenesis to probe the structural differences between APs that absorb in the visible region and FRL-APs and to identify residues essential for the FRL absorbance phenotype. Conversely, red light-absorbing allophycocyanin-B (AP-B; ~ 670 nm) from the same organism was used as a platform for creating a FRL-AP. We demonstrate that the protein environment immediately surrounding pyrrole ring A of PCB on the alpha subunit is mostly responsible for the FRL absorbance of FRL-APs. We also show that interactions between PCBs bound to alpha and beta subunits of adjacent protomers in trimeric AP complexes are responsible for a large bathochromic shift of about ~ 20 nm and notable sharpening of the long-wavelength absorbance band.











Similar content being viewed by others
References
Becraft ED, Wood JM, Rusch DB et al (2015) The molecular dimension of microbial species: 1. Ecological distinctions among, and homogeneity within, putative ecotypes of Synechococcus inhabiting the cyanobacterial mat of Mushroom Spring, Yellowstone National Park. Front Microbiol 6:590. https://doi.org/10.3389/fmicb.2015.00590
Biswas A, Vasquez YM, Dragomani TM et al (2010) Biosynthesis of cyanobacterial phycobiliproteins in Escherichia coli: chromophorylation efficiency and specificity of all bilin lyases from Synechococcus sp. strain PCC 7002. Appl Environ Microbiol 76:2729–2739. https://doi.org/10.1128/AEM.03100-09
Brejc K, Ficner R, Huber R, Steinbacher S (1995) Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution. J Mol Biol 249:424–440. https://doi.org/10.1006/jmbi.1995.0307
Bryant DA, Canniffe DP (2018) How nature designs light-harvesting antenna systems: design principles and functional realization in chlorophototrophic prokaryotes. J Phys B 51:033001. https://doi.org/10.1088/1361-6455/aa9c3c
Bryant DA, Glazer AN, Eiserling FA (1976) Characterization and structural properties of the major biliproteins of Anabaena sp. Arch Microbiol 110:61–75. https://doi.org/10.1007/BF00416970
Bryant DA, Guglielmi G, de Marsac NT et al (1979) The structure of cyanobacterial phycobilisomes: a model. Arch Microbiol 123:113–127. https://doi.org/10.1007/BF00446810
Bryant DA, Rhiel E, De Lorimier R, et al (1990) Analysis of phycobilisome and photosystem I complexes of cyanobacteria. In: Baltscheffsky M (ed) Current research in photosynthesis: Proceedings of the VIIIth international conference on photosynthesis Stockholm, Sweden, August 6–11, 1989. Springer Netherlands, Dordrecht, pp 961–969
Bryant DA, Hunter CN, Warren MJ (2020a) Biosynthesis of the modified tetrapyrroles—the pigments of life. J Biol Chem 295:6888–6925. https://doi.org/10.1074/jbc.REV120.006194
Bryant DA, Shen G, Turner GM et al (2020b) Far-red light allophycocyanin subunits play a role in chlorophyll d accumulation in far-red light. Photosynth Res 143:81–95. https://doi.org/10.1007/s11120-019-00689-8
Capuano V, Thomas JC, Tandeau de Marsac N, Houmard J (1993) An in vivo approach to define the role of the LCM, the key polypeptide of cyanobacterial phycobilisomes. J Biol Chem 268:8277–8283
Chang L, Liu X, Li Y et al (2015) Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res 25:726–737. https://doi.org/10.1038/cr.2015.59
Crooks GE, Hon G, Chandonia J-M, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14:1188–1190. https://doi.org/10.1101/gr.849004
Dong C, Tang A, Zhao J et al (2009) ApcD is necessary for efficient energy transfer from phycobilisomes to photosystem I and helps to prevent photoinhibition in the cyanobacterium Synechococcus sp. PCC 7002. Biochim Biophys Acta 1787:1122–1128. https://doi.org/10.1016/j.bbabio.2009.04.007
Dürring M, Huber R, Bode W et al (1990) Refined three-dimensional structure of phycoerythrocyanin from the cyanobacterium Mastigocladus laminosus at 2.7 Å. J Mol Biol 211:633–644. https://doi.org/10.1016/0022-2836(90)90270-v
Ficner R, Huber R (1993) Refined crystal structure of phycoerythrin from Porphyridium cruentum at 0.23-nm resolution and localization of the γ subunit. Eur J Biochem 218:103–106. https://doi.org/10.1111/j.1432-1033.1993.tb18356.x
Ficner R, Lobeck K, Schmidt G, Huber R (1992) Isolation, crystallization, crystal structure analysis and refinement of B-phycoerythrin from the red alga Porphyridium sordidum at 2.2 Å resolution. J Mol Biol 228:935–950. https://doi.org/10.1016/0022-2836(92)90876-l
Gan F, Bryant DA (2015) Adaptive and acclimative responses of cyanobacteria to far-red light. Environ Microbiol 17:3450–3465. https://doi.org/10.1111/1462-2920.12992
Gan F, Zhang S, Rockwell NC et al (2014) Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345:1312–1317. https://doi.org/10.1126/science.1256963
Gantt E, Lipschultz CA (1973) Energy transfer in phycobilisomes from phycoerythrin to allophycocyanin. Biochim Biophys Acta 292:858–861. https://doi.org/10.1016/0005-2728(73)90036-4
Gibson DG, Young L, Chuang R-Y et al (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. https://doi.org/10.1038/nmeth.1318
Gindt YM, Zhou J, Bryant DA, Sauer K (1992) Core mutations of Synechococcus sp. PCC 7002 phycobilisomes: a spectroscopic study. J Photochem Photobiol B 15:75–89. https://doi.org/10.1016/1011-1344(92)87007-V
Glauser M, Bryant DA, Frank G et al (1992) Phycobilisome structure in the cyanobacteria Mastigocladus laminosus and Anabaena sp. PCC 7120. Eur J Biochem 205:907–915. https://doi.org/10.1111/j.1432-1033.1992.tb16857.x
Glazer AN (1989) Light guides. Directional energy transfer in a photosynthetic antenna. J Biol Chem 264:1–4
Glazer AN, Bryant DA (1975) Allophycocyanin B (λmax 671, 618 nm). Arch Microbiol 104:15–22. https://doi.org/10.1007/BF00447294
Herrera-Salgado P, Leyva-Castillo LE, Ríos-Castro E, Gómez-Lojero C (2018) Complementary chromatic and far-red photoacclimations in Synechococcus ATCC 29403 (PCC 7335). I: the phycobilisomes, a proteomic approach. Photosynth Res 138:39–56. https://doi.org/10.1007/s11120-018-0536-6
Ho M-Y, Gan F, Shen G, Bryant DA (2017a) Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: II. Characterization of phycobiliproteins produced during acclimation to far-red light. Photosynth Res 131:187–202. https://doi.org/10.1007/s11120-016-0303-5
Ho M-Y, Niedzwiedzki DM, MacGregor-Chatwin C et al (2019) Extensive remodeling of the photosynthetic apparatus alters energy transfer among photosynthetic complexes when cyanobacteria acclimate to far-red light. Biochim Biophys Acta 1861:148064. https://doi.org/10.1016/j.bbabio.2019.148064
Ho M-Y, Soulier NT, Canniffe DP et al (2017b) Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr Opin Plant Biol 37:24–33. https://doi.org/10.1016/j.pbi.2017.03.006
Kronfel CM, Kuzin AP, Forouhar F et al (2013) Structural and biochemical characterization of the bilin lyase CpcS from Thermosynechococcus elongatus. Biochemistry 52:8663–8676. https://doi.org/10.1021/bi401192z
Kumar V, Sonani RR, Sharma M et al (2016) Crystal structure analysis of C-phycoerythrin from marine cyanobacterium Phormidium sp. A09DM. Photosynth Res 129:17–28. https://doi.org/10.1007/s11120-016-0259-5
Ledermann B, Aras M, Frankenberg-Dinkel N (2017) Biosynthesis of cyanobacterial light-harvesting pigments and their assembly into phycobiliproteins. In: Hallenbeck PC (ed) Modern topics in the phototrophic prokaryotes: metabolism, bioenergetics, and omics. Springer International Publishing, Berlin, pp 305–340
Li Y, Lin Y, Garvey CJ et al (2016) Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris. Biochim Biophys Acta BBA 1857:107–114. https://doi.org/10.1016/j.bbabio.2015.10.009
Li Y, Lin Y, Loughlin PC, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris – a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 5:67. https://doi.org/10.3389/fpls.2014.00067
Liu JY, Jiang T, Zhang JP, Liang DC (1999) Crystal structure of allophycocyanin from red algae Porphyra yezoensis at 2.2-Å resolution. J Biol Chem 274:16945–16952. https://doi.org/10.1074/jbc.274.24.16945
Liu H, Zhang H, Niedzwiedzki DM et al (2013) Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 342:1104–1107. https://doi.org/10.1126/science.1242321
Ma J, You X, Sun S et al (2020) Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature 579:146–151. https://doi.org/10.1038/s41586-020-2020-7
MacColl R (2004) Allophycocyanin and energy transfer. Biochim Biophys Acta 1657:73–81. https://doi.org/10.1016/j.bbabio.2004.04.005
Madeira F, Park Y, Buso N et al (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. https://doi.org/10.1093/nar/gkz268
Miao D, Ding W-L, Zhao B-Q et al (2016) Adapting photosynthesis to the near-infrared: non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC 7335. Biochim Biophys Acta 1857:688–694. https://doi.org/10.1016/j.bbabio.2016.03.033
Nowack S, Olsen MT, Schaible G et al (2015) The molecular dimension of microbial species. 2. Synechococcus strains representative of putative ecotypes inhabiting different depths in the Mushroom Spring microbial mat exhibit different adaptive and acclimative responses to light. Front Microbiol 6:626. https://doi.org/10.3389/fmicb.2015.00626
Ohkubo S, Miyashita H (2017) A niche for cyanobacteria producing chlorophyll f within a microbial mat. ISME J 11:2368–2378. https://doi.org/10.1038/ismej.2017.98
Olsen MT, Nowack S, Wood JM et al (2015) The molecular dimension of microbial species: 3. Comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat. Front Microbiol 6:604. https://doi.org/10.3389/fmicb.2015.00604
Peng PP, Dong LL, Sun YF et al (2014) The structure of allophycocyanin B from Synechocystis PCC 6803 reveals the structural basis for the extreme redshift of the terminal emitter in phycobilisomes. Acta Crystallogr D 70:2558–2569. https://doi.org/10.1107/S1399004714015776
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738. https://doi.org/10.1038/nprot.2010.5
Schirmer T, Bode W, Huber R et al (1985) X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol 184:257–277. https://doi.org/10.1016/0022-2836(85)90379-1
Schirmer T, Bode W, Huber R (1987) Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 Å resolution. A common principle of phycobilin-protein interaction. J Mol Biol 196:677–695. https://doi.org/10.1016/0022-2836(87)90040-4
Schirmer T, Huber R, Schneider M et al (1986) Crystal structure analysis and refinement at 2.5 Å of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. The molecular model and its implications for light-harvesting. J Mol Biol 188:651–676. https://doi.org/10.1016/s0022-2836(86)80013-4
Sidler WA (1994) Phycobilisome and phycobiliprotein structures. In: Bryant DA (ed) The molecular biology of cyanobacteria. Springer, Dordrecht, pp 139–216
Singh NK, Sonani RR, Rastogi RP, Madamwar D (2015) The phycobilisomes: an early requisite for efficient photosynthesis in cyanobacteria. EXCLI J 14:268–289. https://doi.org/10.17179/excli2014-723
Soulier N, Laremore TN, Bryant DA (2020) Characterization of cyanobacterial allophycocyanins absorbing far-red light. Photosynth Res 145(3):189–207. https://doi.org/10.1007/s11120-020-00775-2
Stec B, Troxler RF, Teeter MM (1999) Crystal structure of C-phycocyanin from Cyanidium caldarium provides a new perspective on phycobilisome assembly. Biophys J 76:2912–2921. https://doi.org/10.1016/S0006-3495(99)77446-1
Tang K, Ding W-L, Höppner A et al (2015) The terminal phycobilisome emitter LCM: a light harvesting pigment with a phytochrome chromophore. Proc Natl Acad Sci USA 112:15880–15885
Wang XQ, Li LN, Chang WR et al (2001) Structure of C-phycocyanin from Spirulina platensis at 2.2 Å resolution: a novel monoclinic crystal form for phycobiliproteins in phycobilisomes. Acta Crystallogr D 57:784–792. https://doi.org/10.1107/s0907444901004528
Xu Q-Z, Han J-X, Tang Q-Y et al (2016) Far-red light photoacclimation: chromophorylation of FR induced α- and β-subunits of allophycocyanin from Chroococcidiopsis thermalis sp. PCC 7203. Biochim Biophys Acta 1857:1607–1616. https://doi.org/10.1016/j.bbabio.2016.06.008
Xu Q-Z, Tang Q-Y, Han J-X et al (2017) Chromophorylation (in Escherichia coli) of allophycocyanin B subunits from far-red light acclimated Chroococcidiopsis thermalis sp. PCC 7203. Photochem Photobiol Sci 16:1153–1161. https://doi.org/10.1039/c7pp00066a
Yu L-J, Kawakami T, Kimura Y, Wang-Otomo Z-Y (2016) Structural basis for the unusual QY red-shift and enhanced thermostability of the LH1 complex from Thermochromatium tepidum. Biochemistry 55:6495–6504. https://doi.org/10.1021/acs.biochem.6b00742
Yu L-J, Suga M, Wang-Otomo Z-Y, Shen J-R (2018) Structure of photosynthetic LH1-RC supercomplex at 1.9 Å resolution. Nature 556:209–213. https://doi.org/10.1038/s41586-018-0002-9
Zhang J, Ma J, Liu D et al (2017) Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551:57–63. https://doi.org/10.1038/nature24278
Zhao C, Gan F, Shen G, Bryant DA (2015) RfpA, RfpB, and RfpC are the master control elements of far-red light photoacclimation (FaRLiP). Front Microbiol 6:1303. https://doi.org/10.3389/fmicb.2015.01303
Zhao J, Zhou J, Bryant D (1992) Energy transfer processes in phycobilisomes as deduced from analyses of mutants of Synechococcus sp PCC 7002. In: Murata N (ed) Research in photosynthesis. Kluwer, Dordrecht, pp 25–32
Acknowledgements
DAB gratefully acknowledges support from the U. S. National Science Foundation (MCB-1613022). This research was also conducted under the auspices of the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the DOE, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC 0001035 (DAB). Molecular graphics and analyses performed with UCSF-Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, were supported by NIH award P410GM103311. The authors would like to thank two anonymous reviewers for their thoughtful reviews and comments that helped us to improve this manuscript. The authors thank Dr. Amy Boal for use of her FPLC system and Gavin Palowitch for performing size-exclusion chromatographic analysis of protein samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial, financial, or non-financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soulier, N., Bryant, D.A. The structural basis of far-red light absorbance by allophycocyanins. Photosynth Res 147, 11–26 (2021). https://doi.org/10.1007/s11120-020-00787-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00787-y