Abstract
Pathways of intramolecular conversion and intermolecular electronic excitation energy transfer (EET) in the photosynthetic apparatus of purple bacteria remain subject to debate. Here we experimentally tested the possibility of EET from the bacteriochlorophyll (BChl) Soret band to the singlet S2 level of carotenoids using femtosecond pump–probe measurements and steady-state fluorescence excitation and absorption measurements in the near-ultraviolet and visible spectral ranges. The efficiency of EET from the Soret band of BChl to S2 of the carotenoids in light-harvesting complex LH2 from the purple bacterium Ectothiorhodospira haloalkaliphila appeared not to exceed a few percent.





Similar content being viewed by others
Abbreviations
- BChl:
-
Bacteriochlorophyll a
- Car(s):
-
Carotenoid(s)
- EET:
-
Excitation energy transfer
- E Car−BChl :
-
Efficiency of EET from carotenoids to BChl
- E Soret−Car :
-
Efficiency of EET from BChl (Soret) to carotenoids
- LH2:
-
Light-harvesting complex 2
- RC:
-
Reaction center
References
Amarie S, Lupo D, Lenz MO, Saegesser R, Ghosh R, Wachtveitl J (2010) Excitation energy pathways in the photosynthetic units of reaction center LM- and H-subunit deletion mutants of Rhodospirillum rubrum. Photosynth Res 103:141–151
Ashikhmin A, Makhneva Z, Moskalenko (2014) The LH2 complexes are assembled in the cells of purple sulfur bacterium Ectothiorhodospira haloalkaliphila with inhibition of carotenoid biosynthesis. Photosynth Res 119:291–303
Cong H, Niedzwiedzki DM, Gibson GN, LaFountain AM, Kelsh RM, Gardiner AT, Cogdell RJ, Frank HA (2008) Ultrafast time-resolved carotenoid-to-bacteriochlorophyll energy transfer in LH2 complexes from photosynthetic bacteria. J Phys Chem B 112:10689–10703
Frank HA, Polívka T (2008) Energy transfer from carotenoids to bacteriochlorophylls. In: Hunter CN, Daldal F, Thurmauer MC, Beatty JT (eds) The purple phototrophic bacteria: Advances in photosynthesis and respiration, vol 28, Springer, Dordrecht, pp 213–230
Fujii R, Shimonaka S, Uchida N, Gardiner A, Cogdell R, Sugisaki M, Hashimoto H (2008) Construction of hybrid photosynthetic units using peripheral and core antennae from two different species of photosynthetic bacteria: detection of the energy transfer from bacteriochlorophyll a in LH2 to bacteriochlorophyll b in LH1. Photosynth Res 95:327–337
Götze JP, Kroner D, Banerjee S, Karasulu B, Thiel W (2014) Carotenoids as a shortcut for chlorophyll soret-to-Q band energy flow. Chem Phys Chem 15:3392–3401
Gradinaru CC, Kennis JTM, Papagiannakis E, van Stokkum IHM, Cogdell RJ, Fleming GR, Niederman RA, van Grondelle R (2001) An unusual pathway of excitation energy deactivation in carotenoids: singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc Natl Acad Sci USA 98:2364–2369
Imhoff JF, Süling J (1996) The phylogenetic relationship among Ectothiorhodospiraceae: a reevaluation of their taxonomy on the basis of 16 S rDNA analyses. Arch Microbiol 165:106–113
Imhoff JF, Trüper HG (1977) Ectothiorhodospira halochloris sp. nov., a new extremely halophilic phototrophic bacterium containing bacteriochlorophyll b. Arch Microbiol 114:115–121
Kosumi D, Maruta S, Horibe T, Fujii R, Sugisaki M, Cogdell RJ, Hashimoto H (2011) Ultrafast energy-transfer pathway in a purple-bacterial photosynthetic core antenna, as revealed by femtosecond time-resolved spectroscopy. Angewandte Chemie International Edition 50:1097–1100
Koyama Y, Rondonuwu FS, Fujii R, Watanabe Y (2004) Light-harvesting function of carotenoids in photo-synthesis: the roles of the newly found 11Bu – state. Biopolymers 74:2–18
Krikunova M, Kummrow A, Voigt B, Rini M, Lokstein H, Moskalenko A, Scheer H, Razjivin A, Leupold D (2002) Fluorescence of native and carotenoid-depleted LH2 from Chromatium minutissimum, originating from simultaneous two-photon absorption in the spectral range of the presumed (optically “dark”) S1 state of carotenoids. FEBS Lett 528:227–229
Limantara L, Fujii R, Zhang J-P, Kakuno T, Hara H, Kawamori A, Yagura T, Cogdell RJ, Koyama Y (1998) Generation of triplet and cation-radical bacteriochlorophyll a in carotenoidless LH1 and LH2 antenna complexes from Rhodobacter sphaeroides. BioChemistry 37:17469–17486
Macpherson AN, Arellano JB, Fraser NJ, Cogdell RJ, Gillbro T (2001) Efficient energy transfer from the carotenoid S2 state in a photosynthetic light-harvesting complex. Biophys J 80:923–930
Magdaong NM, LaFountain AM, Greco JA, Gardiner AT, Carey A-M, Cogdell RJ, Gibson GN, Birge RR, Frank HA (2014) High efficiency light harvesting by carotenoids in the LH2 complex from photosynthetic bacteria: unique adaptation to growth under low-light conditions. J Phys Chem B 118:11172–11189
Magdaong NM, LaFountain AM, Hacking K, Niedzwiedzki DM, Gibson GN, Cogdell RJ, Frank HA (2016) Spectral heterogeneity and carotenoid-to-bacteriochlorophyll energy transfer in LH2 light-harvesting complexes from Allochromatium vinosum. Photosynth Res 127:171–187
Makhneva Z, Bolshakov M, Moskalenko A (2008) Heterogeneity of carotenoid content and composition in LH2 of the sulphur purple bacterium Allochromatium minutissimum grown under carotenoid-biosynthesis inhibition. Photosynth Res 98:633–641
Moskalenko AA, Erokhin YE (1974) Isolation of pigment–lipoprotein complexes from purple photosynthesizing bacteria by the method of preparative polyacrylamide gel electrophoresis. Mikrobiology 43:654–658 (In Russian)
Moskalenko AA, Makhneva ZK (2012) Light-harvesting complexes from purple sulfur bacteria Allochromatium minutissimum assembled without carotenoids. J Photochem Photobiol 108:1–7
Moskalenko AA, Britton G, Connor A, Young A, Toropygina O (1991) The carotenoid content in the chromatophores and pigment–protein complexes isolated from cells of Chromatium minutissimum. Biol Membr USSR 8:249–260 (in Russian)
Moskalenko AA, Toropygina OA, Makhneva ZK (1997) Behavior of carotenoids in Rhodospirillum rubrum cells under cultivation with diphenylamine. Dokl Akad Nauk 355:259–261 (Russian)
Niedzwiedzki DM, Fuciman M, Kobayashi M, Frank HA, Blankenship RE (2011) Ultrafast time-resolved spectroscopy of the light-harvesting complex 2 (LH2) from the photosynthetic bacterium Thermochromatium tepidum. Photosynth Res 110:49–60.
Polívka T, Frank HA (2010) Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc Chem Res 43:1125–1134
Polívka T, Sundström V (2004) Ultrafast dynamics of carotenoid excited states–from solution to natural and artificial systems. Chem Rev 104:2021–2072
Rademaker H, Hoff AJ, van Grondelle R, Duysens LNM (1980) Carotenoid triplet yields in normal and deuterated Rhodospirillum rubrum. Biochim et Biophys Acta 592:240–257
Scheer H (2006) An overview of chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. In: Grimm В et al. (eds) Chlorophylls and bacteriochlorophylls. Springer, Berlin, Chap. 1, pp 1–26
Slouf V, Fuciman M, Dulebo A, Kaftan D, Koblízek M, Frank HA, Polívka T (2013) Carotenoid charge transfer states and their role in energy transfer processes in LH1–RC complexes. J Phys Chem B 117:10987–10999
Sundström V (2008) Femtobiology. Annu Rev Phys Chem 59:53–77
Theiss C, Leupold D, Moskalenko AA, Razjivin AP, Eichler HJ, Lokstein H (2008) Femtosecond spectroscopy of native and carotenoidless purple-bacterial LH2 clarifies functions of carotenoids. Biophys J 94:4808–4811
Tretiak S, Middleton C, Chernyak V, Mukamel S (2000) Bacteriochlorophyll and carotenoid excitonic couplings in the LH2 system of purple bacteria. J Phys Chem B 104:9540–9553
Acknowledgements
This work has been supported in part by grants from the Russian Foundation for Basic Research (15-04-09289-a; OFI-M 15-29-01167; 15-04-02660-a). We are thankful to Dr. E. A. Kotova for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
We estimate the degree of sample bleaching when short laser pulses λ = 350 nm pass through the rotating cell with L = 1.5 mm layer of LH2 preparation.
Excitation laser pulse energy was measured to be W = 0.24 µJ, it thus including n0 = W/(hc/λ) = 0.41 × 1012 quanta. Since absorption at 340 nm is D340 = 0.350, the number of quanta absorbed in the cell is n = n0·(1–10−D340) = 0.227 × 1012. These quanta were absorbed within the cylindrical volume containing the LH2 sample; since the cylinder diameter d = 0.3 mm, the illuminated cylinder volume V = 1.06 × 10−7 liter. Although the exact composition of the LH2 complex of Ect. haloalkaliphila is not known, it is assumed to be similar to LH2 composition of Rps. acidophila, which includes p = 18 molecules of BChl B850, q = 9 molecules of BChl B800, and r = 9 molecules of Cars.
Only BChl B850 molecules absorb light at 846 nm, so with the known molar extinction coefficient ε = 1.33 × 105 and measured absorbance D846 = 0.457 we calculate the molar concentration of BChl B850 in the sample, CB850 = D846/(ε846·L) = 22.9 × 10−6 M, and the number of BChl B850 and B800 molecules in the illuminated volume: NB850 = CB850·NA·V = 1.46 × 1012, NA being Avogadro’s number, NB800 = NB850·q/p = 0.731 × 1012, total BChl number NBChl = NB850 + NB800 = 2.19 × 1012. Similarly, Car molecules number NC = NB850·r/p = 0.731 × 1012.
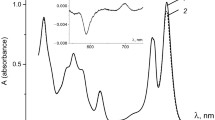
Both BChl and Car molecules contribute to the absorbance at 340 nm, the contribution of Car molecules being about β = 0.05 (see Fig. 4). This means that nC = n·β = 1.14 × 1010 quanta were absorbed by Car molecules, while the remaining nB = n·(1−β) = 2.16 × 1011 quanta were absorbed by BChl molecules.
Absorption of excitation quanta by some Car molecules appears as a proportional reduction in the Car absorption band centered at 500 nm, where the LH2 preparation absorbance was measured to be D500 = 0.287. Obviously, the excitation of fC = FC/nC = 0.0155 fraction of Car molecules would result in ΔD500 = fC·D500 = 0.00446 bleaching at 500 nm. Indeed, the calculated bleaching ΔD500 = 0.00446 is close to the experimentally measured value ΔA500 = 0.00416, differing by only 6–7%.
Rights and permissions
About this article
Cite this article
Razjivin, A.P., Lukashev, E.P., Kompanets, V.O. et al. Excitation energy transfer from the bacteriochlorophyll Soret band to carotenoids in the LH2 light-harvesting complex from Ectothiorhodospira haloalkaliphila is negligible. Photosynth Res 133, 289–295 (2017). https://doi.org/10.1007/s11120-017-0341-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0341-7