Abstract
Background and aims
Biological nitrification inhibition (BNI) is a chemical ecological phenomenon whereby plants specifically suppress nitrification by releasing inhibiting compounds from roots, an effective strategy for improving nitrogen uptake by limiting nitrogen losses from agricultural fields. During this study, we have aimed at characterizing hydrophilic BNI activity released from maize roots to understand the chemical basis for BNI function in maize.
Methods
Maize plants were grown hydroponically and root exudates were collected using water-based solutions and hydrophilic BNI activity was extracted. We isolated the target BNI compounds by a combination of chromatographic techniques and bioassays using a recombinant luminescent ammonia-oxidizing bacterium Nitrosomonas europaea (pHLUX20).
Results
We identified 6-methoxy-2(3H)-benzoxazolone (MBOA) as the responsible BNI compound with a median effective dose (ED50) = 0.76 μM. MBOA inhibited the conversion of NH3 to NH2OH as well as NH2OH to NO2− in N. europaea, suggesting that MBOA blocks both ammonia monooxygenase and hydroxylamine oxidoreductase enzymatic pathways. Treatment with MBOA significantly suppressed NO2− and NO3− production during soil incubation, but this activity was reduced subsequently due to biodegradation of MBOA by soil microbes. A quantification experiment revealed that MBOA accounted for nearly 50% of the total BNI activity in hydrophilic and hydrophobic exudates from maize roots. A soil incubation test showed that two previously identified benzoxazinoids, HDMBOA and HDMBOA-β-glucoside, can be eventually transformed into MBOA.
Conclusion
We elucidated MBOA as the key component of BNI in maize. Collectively, the present findings will serve as the groundwork for construction of an advanced environment-friendly agricultural system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In agriculture, large amounts of nitrogen fertilizers are used for crop production to feed the growing world population. Nearly 50% of the nitrogen fertilizer applied to Poaceae crops (e.g., maize, wheat, and rice) is lost largely because of two soil microbial transformations, nitrification and denitrification, resulting in loss of soil-nitrogen and low nitrogen use efficiency (NUE) (Coskun et al. 2017; Subbarao et al. 2013b; Thakur and Medhi 2019). Nitrification, a stepwise oxidation process from NH3 to nitrate (NO3−) by soil microbes, plays an important role in the nitrogen cycle. However, excessive production of NO3− and its high mobility leads to groundwater contamination and generation of harmful greenhouse gas such as N2O and NO (Kuypers et al. 2018; Rivett et al. 2008; Scheer et al. 2020; Stayner et al. 2017; Tian et al. 2020). Thus, overapplication of nitrogen fertilizer (as NH3) together with generation of excess soil-NO3− provoke serious economic and environmental damage (Subbarao and Searchinger 2021). Therefore, a strategy is needed for suppression of nitrification to increase NUE to correct the imbalances in nitrogen cycle. Biological nitrification inhibition (BNI) provides an innovative way to reduce nitrogen loss from agricultural system (Ghatak et al. 2022; Subbarao and Searchinger 2021). BNI is a chemical ecological phenomenon by which specific natural products (secondary metabolites) secreted from the plant root system, including terpenoids, alkaloids, fatty acids, and phenylpropanoids, that inhibit nitrification and growth of nitrifiers (Subbarao et al. 2009; Subbarao et al. 2013b; Wendeborn 2020). The BNI-possessing crops can retain more NH4 in the rhizosphere through circumvention of NO3− production. Therefore, utilization of BNI has several major advantages. (a) It is eco-friendly: the environmental pollution risk is reduced through using the activity of phytochemicals released from crop root systems. (b) The effect is sustainable: plant crops can continuously biosynthesize and secrete BNI compounds from roots into rhizosphere to keep nitrifier activity under check. (c) Costs are reduced: application of additional agrochemicals is not needed. To date, BNI has been observed in certain plants, including main staple food crops, namely maize, wheat, rice, and sorghum (Otaka et al. 2022; Subbarao et al. 2021; Subbarao et al. 2013a; Sun et al. 2016; Zakir et al. 2008). Understanding BNI function in maize is important due to: (a) Among staple crops, maize is the most productive crop providing food and feed, (b) It consumes a major portion of nitrogen fertilizer produced globally, and (c) Maize production systems contribute to nitrogen pollution in a major way globally.
To develop a BNI strengthened crop with a higher yield of BNI compounds, understanding the chemical identity of BNI compounds released from roots is essential. For isolation of BNI compounds, a crucial property is whether the root exudates and compounds are water-insoluble (hydrophobic) or water-soluble (hydrophilic) (Subbarao et al. 2013a). Water-insoluble hydrophobic compounds with lower mobility are predominant in the rhizosphere, whereas hydrophilic compounds in water can move more farther from the roots and have wide-reaching influence. Chemical identities of both hydrophobic and hydrophilic BNI compounds in plant crops will lead to a deeper understanding of BNI function in the soil. We have recently reported two major hydrophobic BNI-contributing compounds from the root surface in maize, namely 2,7-dimethoxy-1,4-naphthoquinone (zeanone; ED50 = 2 μM) and 2-hydroxy-4,7-dimethoxy-2H-1,4-benzoxazin-3(4H)-one (HDMBOA; ED50 = 13 μM), together with two analogs of HDMBOA from inside the roots, namely 7-methoxy-2H-1,4-benzoxazin-3(4H)-one (HMBOA; ED50 = 91 μM) and HDMBOA-β-glucoside (ED50 = 94 μM) (Fig. S1) (Otaka et al. 2022). This study is aimed at further understanding of the BNI function in maize root systems by focusing on chemical characterization of hydrophilic BNI activity that include (a) Isolation and structural determination of BNI-active components, (b) Determination of mode of inhibitory action of the isolated BNI compounds, and (c) Confirmation of BNI function of isolated compounds using in vitro and in vivo approaches.
Materials and methods
Hydroponic cultivation of maize
Ninety seeds of maize ‘Honey Bantam’ (Sakata Seed Corp., Kanagawa, Japan) were wrapped in a moistened paper towel and incubated in a growth chamber in the dark at 25 °C for 24 h (Fig. S2a). The seeds were then soaked in a plastic bottle containing 200 μM CaSO4 aqueous solution (aq.) (200 mL) (Fig. S2b). Ten bottles (in total 900 seeds) were prepared and placed in the dark with bubble aeration at 25 °C for 24 h. The seeds were sown in seedling-grow boxes and the seedlings were supplied with 200 μM CaSO4 aq. (Figure S2c). The seedling-grow boxes were placed in a growth chamber maintained at 29 °C for 13 h/25 °C for 11 h (max/min) with average light intensity of ~360 μmol m−2 s−1 (25,000 lx) and relative humidity of 70%.
After 7 days in the seedling-grow boxes, the seedlings were transferred to a hydroponic system with aeration in the growth chamber. The hydroponic system consisted of ten tanks (one tank per 90 L), with each tank having a styrofoam top with eight holes in two rows (Fig. S2d). Six maize plants (one batch) were planted in a hole; in total 480 plants were grown in the ten tanks. The tanks contained nutrient solution with the following composition: KH2PO4 (final concentration: 281 μM), K2SO4 (final concentration: 178 μM), CaCl2·2H2O (final concentration: 71 μM), MgSO4·7H2O (final concentration: 150 μM), Fe-EDTA (final concentration: 41 μM), H3BO3 (final concentration: 922 μM), CuSO4·5H2O (final concentration: 0.3 μM), MnSO4·6H2O (final concentration: 9.1 μM), Na2MoO4·2H2O (final concentration: 5.2 μM), and ZnSO4·7H2O (final concentration: 7.7 μM) dissolved in 100 L deionized water. In addition, NH4NO3 was added to the nutrient solution as the nitrogen source at a final concentration of 1.0 mM. During cultivation for the first 21 days, the pH of the nutrient solution was adjusted daily in the range 6.0–6.5 using 1 N HCl or 1 N NaOH. The nutrient solution was replaced with fresh solution every 7 days.
After 21 days, NH4NO3 as the nitrogen source was changed to ammonium sulfate (NH4)2SO4 in the nutrient solution at a final concentration of 0.5 mM, and the maize plants were cultivated for an additional 7 days.
Collection of root exudates of maize
The 30-day-old maize plants (total 80 batches) (Fig. S2e) were removed from the ten nutrient solution tanks and rinsed with deionized water for 2–3 min. The root exudates of the plants were successively collected with the following four steps (a–d) (Fig. S2f).
-
(a). Each batch was transferred to a 2.0 L plastic bottle containing CaSO4 (final concentration: 200 μM) and NH4Cl (final concentration: 500 μM) in aqueous solution (1.8 L), in which only the roots were soaked, for 24 h with aeration (N1 solution).
-
(b). The maize plants were removed from the N1 solution and their roots were soaked in 200 μM CaSO4 aq. (1.8 L) for 24 h with aeration (W1 solution).
-
(c). The maize plants were removed from the W1 solution and were moved to aqueous solution (1.8 L) containing CaSO4 (final concentration: 200 μM) and NH4Cl (final concentration: 500 μM) for 24 h with aeration (N2 solution).
-
(d). The maize plants were removed from the N2 solution and the roots were soak in 200 μM CaSO4 aq. (1.8 L) for 24 h with aeration (W2 solution).
In total 320 bottles of the four solutions (N1, W1, N2, and W2) were collected and all solutions were combined (total 480 L).
Chemicals and solvents
All chemicals and solvents were purchased from commercial suppliers or prepared as described in this article. Water was purified by a Milli-Q system (Merck Millipore, Billerica, MA, USA) unless indicated otherwise.
Instrumentation
Ultraviolet (UV) spectra were measured with a Shimadzu UV-1200 spectrometer. Nuclear magnetic resonance (NMR) spectra were measured in CDCl3 with a Bruker AVANCE III HD 800 MHz spectrometer. Chemical shifts δ (ppm) were referenced to the residual solvent peaks (δH 7.24 ppm; δC 77.0 ppm), and coupling constants (J) were reported in hertz (Hz). Multiplicity was indicated as follows: s = singlet; d = doublet; dd = doublet of doublet; and brs = broad singlet. Liquid chromatography−electrospray ionization−mass spectrometry (LC-ESI-MS) analyses were conducted on a Shimadzu LCMS-2020 instrument (Shimadzu Corp., Kyoto, Japan) equipped with a TSKgel Super-ODS column (4.6 mm I.D. × 50 mm, 2.3 μm; Tosoh Corp., Tokyo, Japan). The analytical and preparative (prep.) reverse-phase high-performance liquid chromatography (RP-HPLC) was performed on a Shimadzu Prominence LC-20AT instrument (Shimadzu Corp.) with a Shimadzu SPD-M20A photodiode array detector (Shimadzu Corp.), accompanied by a TSKgel Super-ODS column (4.6 mm I.D. × 50 mm, 2.3 μm) or a Cosmosil πNAP column (4.6 mm I.D. × 150 mm, 5.0 μm; Nacalai Tesque Inc., Kyoto, Japan). The optical densities at 660 nm (O.D. 660) was measured on a mini photo 518R photo meter (TAITEC Corp., Nagoya, Japan).
Extraction, separation, and purification of BNI compounds from maize root exudates
The solution containing maize root exudates (480 L) was concentrated under reduced pressure at 35 °C to obtain a brown syrup (24 g). A concentrate (24 g) was partitioned between 30% MeOH/70% H2O (300 mL) and ethyl acetate (EtOAc) (300 mL), followed by concentration of both layers under vacuum at 35 °C to yield a 30% MeOH aqueous fraction (23.4 g) and an EtOAc fraction (580 mg), respectively. The EtOAc fraction (580 mg) was dissolved in 10% MeOH/90% H2O (50 mL), extracted with hexane (50 mL), and both layers were concentrated at 35 °C to obtain a 10% MeOH aqueous fraction (540 mg, fraction M) and a hexane fraction (40 mg), respectively.
Fraction M (540 mg) was separated by RP-HPLC on a TSKgel Super-ODS column using a linear gradient [1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (6 min) to 100% acetonitrile (9 min), 1.0 mL min−1] to give five fractions (M-1–M-5). Fraction M-2 (40 mg, tR 5.5–8.0 min) was separated by RP-HPLC on a πNAP column [30% acetonitrile/70% H2O (0.1% formic acid) (start) to 50% acetonitrile/50% H2O (0.1% formic acid) (10 min), 1.0 mL min−1], affording five subfractions (M-2-1–M-2-5). Fraction M-2-2 (15 mg, tR 6.0–8.0 min) was further fractionated by RP-HPLC on a πNAP column [10% acetonitrile/90% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (25 min) to 100% acetonitrile (30 min), 1.0 mL min−1] to obtain four fractions (M-2-2-1–M-2-2-4). Fraction M-2-2-1 (2.5 mg, tR 18.5–22.0 min) was divided into two fractions by RP-HPLC on a πNAP column [30% acetonitrile/70% H2O (0.1% formic acid), 1.0 mL min−1], and fractions M-2-2-1-2 (tR 7.2–7.8 min) and M-2-2-1-1 (tR 6.8–7.2 min) were dried to isolate compounds 1 (0.5 mg) and 2 (0.7 mg), respectively.
-
Compound 1: colorless crystal (H2O); MS (pos.) m/z 166 [M + H]+, (neg.) m/z 164 [M − H]−; UV (H2O) λmax (log ε) 287 (3.75), 230 (4.01) nm; solubility in water 0.56 mg/mL (25 °C).
-
Compound 2: white powder (MeOH); MS (pos.) m/z 196 [M + H]+; UV (acetonitrile:H2O 1:1) λmax (log ε) 281 (3.58), 231 (4.02), 202 (4.49) nm.
Preparation of calibration curves by using HPLC
The purified samples were dissolved in H2O or MeOH at concentrations ranging from 0 to 100 ppm. Each 5 μL sample solution was analyzed using a Shimadzu LCMS-2020 instrument on a TSKgel Super-ODS column [1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (13 min), 0.4 mL min−1]. Based on the peak area (mV*sec) of each compound, a calibration curve was prepared.
Bioluminescence assay using N. europaea (pHLUX20) [nitrogen source: (NH4)2SO4]
The detailed description of the bioluminescence bioassay was described in our earlier publications (Subbarao et al. 2006). A recombinant N. europaea IFO 14298 (pHLUX20) was used (Iizumi et al. 1998). The composition of the culture medium (P medium) for N. europaea was as follows: (NH4)2SO4 (final concentration: 18.9 mM), KH2PO4 (final concentration: 5.1 mM), Na2HPO4 (final concentration: 95.0 mM), NaHCO3 (final concentration: 6.0 μM), MgSO4·7H2O (final concentration: 405 μM), CaCl2·2H2O (final concentration: 340 μM), Fe-EDTA (final concentration: 2.7 μM), and kanamycin·nH2SO4 (0.25 mg) dissolved in 1.0 L water (Lewis and Pramer 1958). The recombinant N. europaea (pHLUX20) was grown in a 500 mL baffled Erlenmeyer flask containing 200 mL medium at pH 7.8 on a rotary shaker at 150 rpm at 30 °C for 7 days.
After cultivation, bacterial cells from the three flasks (total 600 mL medium) were concentrated by centrifugation at 9000 rpm at 5 °C for 10 min and resuspended in 10 mL P medium (O.D. 660 = 1.0). The bacterial suspension was left in the dark for 10 min at 25 °C. The test sample (extract, fraction, and compound) was dissolved in water or DMSO, from which an aliquot (1.0 μL) was added to 199 μL double-distilled water. To a solution of sample (200 μL) was added 250 μL bacterial culture, then the mixture (total 450 μL) was incubated at 15 °C for 20 min. An aliquot of each resulting mixture (100 μL) was added to 2.5 μL of 10% n-decyl aldehyde in ethanol, and the bioluminescence of the sample was measured on a GLOMAX 20/20 Luminometer (Promega Corp., Madison, WI, USA). To test the bacterial condition, fluorescence in N. europaea (pHLUX20) was preliminary checked using 0.22 μM allylthiourea as a positive control to show approximately 80% inhibition. Each sample was measured at least three times, and the average value was used to calculate the BNI activity. The BNI activity of each sample is expressed in allylthiourea units (ATU), where 1 ATU means 80% inhibition by allylthiourea on bioluminescence (0.22 μM) (Subbarao et al. 2006).
Bioluminescence assay using N. europaea (pHLUX20) [nitrogen source: (NH2OH)]
A concentrated culture broth of 7-day-old N. europaea (pHLUX20) (600 mL) was washed twice with P medium lacking (NH4)2SO4 and then resuspended with 10 mL P medium containing NH2OH (final concentration: 10 μM) as the sole nitrogen source (O.D. 660 = 1.0). In the same manner as described for the “Bioluminescence assay using N. europaea (pHLUX20) [nitrogen source: (NH4)2SO4]”, the BNI activity of the test sample was measured as described earlier (Iizumi et al. 1998; Subbarao et al. 2006).
Effect of the BNI compound on NO2 − production by N. europaea (pHLUX20)
To an aqueous solution of the BNI compound (1.0 mL, final concentration: 2.0–50 μM) was added P medium (100 mL) containing (NH4)2SO4 (final concentration: 750 μM) or NH2OH (final concentration: 20 μM) in a 500 mL baffled Erlenmeyer flask. Instead of the BNI compound, water (1.0 mL) was added to medium as a control. A 250 μL aliquot of concentrated 7-day-old N. europaea (pHLUX20) suspension (O.D. 660 = 1.0), which was washed twice by P medium lacking a nitrogen source [(NH4)2SO4 and NH2OH], was inoculated into each medium and incubated on a rotary shaker at 150 rpm at 30 °C. An aliquot of the reaction mixture at 0 min (inoculated time), 1 h, and 3 h after inoculation was collected from each flask. The concentration of NO2− was measured using the NO2/NO3 Assay Kit-C II (Colorimetric) Griess Reagent Kit (NO2− concertation range: 10–100 μM) (Dojindo Co., Kyoto, Japan) or the NO2/NO3 Assay Kit-FX (Fluorometric) 2,3-Diaminonaphthalene (DAN) Kit (NO2− concertation range: 1.0–10 μM) (Dojindo Co.) in accordance with the manufacturer’s protocol. The absorbance of the Griess products in the test samples were measured at 540 nm, and the fluorescence of the DAN products were measured at λex = 360 nm/λem = 450 nm, both of which were conducted on an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, USA). The concentration of NH4+ was measured using the LabAssay™ Ammonia Kit (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), for which the absorbance of the indophenol blue products was measured at 630 nm. Each experiment (Griess reaction, DAN reaction, and indophenol blue reaction) was replicated three times.
Effect of the BNI compound on growth of N. europaea (pHLUX20)
A 7-day-old N. europaea (pHLUX20) suspension (250 μL, O.D. 660 = 1.0) was inoculated into 100 mL P medium containing (NH4)2SO4 (final concentration: 18.9 mM) as the nitrogen source and the BNI compound dissolved in H2O (1.0 mL, final concentration: 10 μM) in a 500 mL baffled Erlenmeyer flask. Instead of the BNI compound, water (1.0 mL) was used as a blank control. Each medium was incubated on a rotary shaker at 150 rpm at 30 °C. An aliquot of culture medium (3.0 mL) was subsequently collected from each flask at 0 min (inoculated time), day 1, day 3, and day 5 after inoculation. The value of O.D. 660 for each sample was corrected by that of the blank control containing only P medium.
To analyze the stability of the BNI compound in the bacterial culture, collected samples were centrifuged at 7000 rpm for 10 s before HPLC. Each 5 μL of supernatant was analyzed by HPLC on a TSKgel Super-ODS column [1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (13 min) to 100% acetonitrile (18 min), 0.4 mL min−1].
Soil incubation test for determining potential nitrification
Potential soil nitrification was determined using a modified soil slurry protocol (Vazquez et al. 2020). The brief protocol is as follows: Soil samples collected from the JIRCAS experimental farm in Tsukuba City were used (Typic Hapludands; pH 6.0 in water; clay 54.8%, silt 26.3%, sand 18.9%; C/N ratio = 29.2/2.5 mg g−1 soil, CEC = 119.1 mEq/100 g). The soil was air-dried at room temperature and sifted through a 2 mm sieve. To 100 mL nitrogen fertilizer solution [KH2PO4 (final concentration: 300 μM), K2HPO4 (final concentration: 700 μM), (NH4)2SO4 (final concentration: 750 μM), water (98 mL)] was added air-dried soil (10 g) in a 500 mL baffled Erlenmeyer flask. After the addition of BNI compounds [0.8 mg (final concentration: 50 μM) and 1.6 mg (final concentration: 100 μM)], each soil solution was sealed with a silico plug and incubated on a rotary shaker at 100 rpm at 20 °C. An aqueous solution of the synthetic nitrification inhibitor dicyandiamide (DCD, final concentration = 327 μM) and water was used as a positive control and blank control, respectively. The sterilized soil was used as a negative control. An aliquot of soil mixture (soil + buffer) at 1 h, day 1, day 2, day 3, day 4, day 5, and day 7 after incubation was collected into a 1.5 mL tube from each flask. After centrifugation of the assay solution at 7000 rpm for 10 s, each supernatant was immediately transferred to a new 1.5 mL tube. All collected samples were stored in a deep-freezer at −80 °C until use. The concentration of NO2− and NO3− in each sample was measured using the NO2/NO3 Assay Kit-C II (Colorimetric) Griess Reagent Kit (Dojindo Co.) or the NO2/NO3 Assay Kit-FX (Fluorometric) 2,3-Diaminonaphthalene Kit (NO2− concertation range: 1.0–10 μM) (Dojindo Co.). To measure the concentration of the BNI compound in the sample, a 5 μL aliquot of the supernatant was analyzed by LC-ESI-MS on a TSKgel Super-ODS column [1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (13 min) to 100% acetonitrile (18 min), 0.4 mL min−1].
Analysis of stability of BNI compounds in soil
Air-dried soil (100 mg) was moistened with 500 μL water in a glass vial. To 5 μL solution of the BNI compound (350 μM), moistened soil was added and the soil mixture was incubated at 25 °C. An aliquot of reactant (20 μL) was collected at 0 min (inoculated time), 1 h, 3 h, 6 h, and 24 h after inoculation. Each collected sample was centrifuged, and the 5 μL supernatant was analyzed by LC-ESI-MS with monitoring at 210 nm [1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (13 min), 0.4 mL min−1]. The sterilized soil was used as a negative control.
Results
Isolation and identification of the hydrophilic BNI compound from root exudates of maize
To explore hydrophilic BNI compounds in exudates from maize roots, we began our study on the basis of the following planned steps (I–IV): I. Hydroponic cultivation of maize; II. Soaking of intact maize roots into aerated CaCl2 aqueous solutions under induction of BNI by NH4Cl; III. Concentration of the combined soaked solutions to obtain hydrophilic root exudates; and IV. Isolation of hydrophilic BNI compounds in the root exudates based on the results of a bioluminescent assay using N. europaea (pHLUX20).
For collection of hydrophilic BNI compounds from maize roots, we concentrated 480 L extraction solution from 30-day-old maize roots to collect root exudates (24 g). Because the hydrophilic root exudates (24 g) contained not only maize metabolites but also other compounds, such as inorganic salts from fertilizer, we partitioned the exudates between EtOAc and 30% MeOH aqueous solutions. In the first bioassay, the EtOAc fraction (580 mg) showed nitrification inhibitory activity against N. europaea, whereas the 30% MeOH layer (19.4 g) containing inorganic salts had no such activity. Hence, we further partitioned the EtOAc fraction between hexane and 10% MeOH aqueous solutions. Because BNI activity was concentrated in the 10% MeOH aqueous fraction (fraction M; 540 mg) (18,256 ATU), we then aimed to purify the BNI compound.
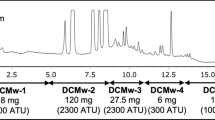
We narrowed down the target BNI fraction, composed of two peaks 1 and 2, through successive purification of fraction M (540 mg) based on a bioassay-guided fractionation method (Fig. 1). Then, we separated and isolated two BNI candidates, 1 (0.5 mg) and 2 (0.7 mg), that showed similar UV spectral patterns (Fig. S3). The structures of both compounds were elucidated as 6-methoxy-2(3H)-benzoxazolone (MBOA, 1) and 6,7-dimethoxy-2(3H)-benzoxazolone (DMBOA, 2) from the NMR (1H and 13C) and MS data, together with comparison of their spectroscopic data with previously reported data in the literature (Figs. 2, S4, and S5, Tables 1 and S1) (Hasegawa et al. 1992; Kosemura et al. 1995; Nagao et al. 1985). The difference in chemical structure between MBOA (1) and DMBOA (2) is the presence of a methoxy group at C-7 (Fig. 2).
Stepwise purification and isolation of the BNI compound. Two candidates 1 and 2 were isolated based on the bioassay-guided fractionation method. Reverse-phase-HPLC analyses of the obtained fractions and compounds were performed on a TSKgel Super-ODS column [injected volume: 5 μL, 1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (13 min) to 100% acetonitrile (18 min), 0.4 mL min−1, at 210 nm]
MBOA (1), which belongs to the benzoxazolone group, was first identified in 1955 from the root of two Coix species, including adlay (Coix lacryma-jobi L.) (Koyama 1955; Koyama and Yamato 1955). In 1957, two groups individually reported the isolation, identification, and biological activities of MBOA (plant-resistance factor and antimicrobial activity) from maize and wheat seedlings (Smissman et al. 1957; Virtanen et al. 1957). DMBOA (2) was first identified from dried maize tissue that appeared to be a degradation product of benzoxazinoids (BXs) (Klun et al. 1970). Several studies on the anti-auxin activity of DMBOA have been reported (Anai et al. 1996; Kosemura et al. 1995; Kosemura et al. 1994; Venis and Watson 1978).
Bioactivity of BNI compound in hydrophilic root exudates of maize
MBOA (1) showed strong BNI activity, whereas the activity of DMBOA (2) was markedly weaker (Fig. 3a). Moreover, a dose–response curve for standard MBOA (FUJIFILM Wako Pure Chemical Corp.) reproduced that of isolated 1, having an ED50 value of 0.76 μM (Fig. 3b). Thus, we elucidated MBOA to be the strongest BNI compound in hydrophilic root exudates of maize. Remarkably, in contrast to other reported BNI compounds, the shape of the dose–response curve for MBOA to N. europaea (pHLUX20) was unprecedented. Previously reported BNI compounds, including zeanone (ED80 = 8 μM), HDMBOA (ED80 = 70 μM), and HDMBOA-β-glucoside (ED80 = 300 μM), displayed a linear correlation between their bioluminescence inhibition and concentration (Fig. S6), from which each ED80 was calculated (Otaka et al. 2022; Subbarao et al. 2009; Subbarao et al. 2013a). In contrast, MBOA displayed more than 50% inhibition in a dosage range of 0.76–300 μM but did not attain 80% inhibition (Figs. 3b and S6). These results indicated that MBOA had a different function in nitrification from those of known nitrification inhibitors.
Dose–response curves of isolated MBOA (1) and DMBOA (2) (a) and that of standard MBOA (b). A 7-day-old pure Nitrosomonas europaea (pHLUX20) culture was treated with the indicated concentrations of MBOA for 20 min. The bioluminescence of the control reaction after the addition of water instead of BNI compounds is defined as 100% bioluminescence. The dose–response curve is a plot of the treated dose (μM) on the X-axis versus the response (percentage inhibition of bioluminescence) on the Y-axis. (b) Error bars indicate the standard deviation of the mean (n = 3). Error bars are not visible as they are smaller than symbols
Mode of action of MBOA on N. europaea
To validate the influence of MBOA in nitrification, we then tested whether MBOA inhibited the oxidation pathway from NH3 (ammonium ion form, NH4+) to NO2− in N. europaea. Two concentrations of MBOA (2.0 and 10 μM) were added to N. europaea (pHLUX20) broth containing (NH4)2SO4 as the sole nitrogen source, which was incubated on a rotary shaker. An aliquot of each bacterial broth was collected at 0 min, 1 h, and 3 h after inoculation. The concentrations of both NO2− and NH4+ in the sample were measured based on the Griess reaction for 10–100 μM NO2−, the DAN reaction for 0.1–10 μM NO2−, and the indophenol blue reaction for NH4+ (Ivancic and Degobbis 1984; Nussler et al. 2006; Tsikas 2007).
MBOA inhibited the production of NO2− from N. europaea, with the inhibition rate ranging from approximately 50% at 2 μM and > 99% at 10 μM at 3 h after inoculation (Fig. 4a). The concentration of NH4+ was maintained by MBOA, indicating that NH4+ consumption was suppressed (Fig. 4b). Similarly, allylthiourea, a commercial synthetic nitrification inhibitor, suppressed NO2− production together with NH4+ consumption (Fig. 4a and b). Hence, these results proved that MBOA could inhibit the AMO pathway in N. europaea.
Time-course analysis of NO2− production (a) and NH4+ consumption (b) from Nitrosomonas europaea (pHLUX20) in the presence of MBOA. A 7-day-old N. europaea (pHLUX20) solution (250 μL, O.D. 660 = 1.0) was inoculated into 100 mL P medium [nitrogen source: (NH4)2SO4)] upon addition of MBOA (2 and 10 μM) and incubated at 150 rpm and 30 °C. Allylthiourea (AT, 10 μM) and water was used as a positive control and control, respectively. After cultivation for 0 min (start: inoculated time), 1 h, and 3 h, an aliquot of the medium was collected to measure the NO2− and NH4+ concentrations. Error bars indicate the standard deviation of the mean (n = 3). Invisible error bars were smaller than symbols
As N. europaea derives vital free energy from AMO-catalyzing oxidation of NH4+ (Prosser 1990), we proposed that MBOA might inhibit the growth of N. europaea. To test this hypothesis, we assessed the effect of MBOA on the growth of N. europaea (pHLUX20) by measuring the O.D. at 660 nm to estimate the concentration of bacteria.
As expected, treatment with MBOA distinctly inhibited the cell growth of N. europaea (pHLUX20) for 5 days after inoculation (Fig. 5a and b). It has been reported that AOB can oxidize a variety of aromatic compounds (Chang et al. 2002; Keener and Arp 1994). In addition, AMO are potent oxidizers of not only NH4+ but also various small molecules (e.g., allylsulfide and C2H4) as substrates that show competitive nitrification inhibition (Juliette et al. 1993; Keener and Arp 1994). To test the stability and potential as a substrate of MBOA against N. europaea, we analyzed a culture of MBOA-treated N. europaea (pHLUX20) for 5 days by RP-HPLC monitored at 287 nm. Consequently, MBOA was not metabolized by N. europaea because the peak area of MBOA did not vary (Fig. S7). These results demonstrated that MBOA alone, but no other metabolized compounds, could suppress AMO activity in N. europaea in addition to cell growth.
Time-course analysis of cell growth (a) and appearance (b) of Nitrosomonas europaea (pHLUX20) in (NH4)2SO4 medium with or without MBOA. A 7-day-old N. europaea suspension (250 μL, O.D. 660 = 1.0) was inoculated to 100 mL P medium containing (NH4)2SO4 (final concentration: 18.9 mM) upon treatment with MBOA (10 μM) and grown on a rotary shaker at 150 rpm at 30 °C for 5 days. Water (1.0 mL) was used instead of MBOA as the control. (a) Cell growth (O.D. 660) was monitored on a mini photo 518R photometer at 0 min (start: inoculated time), day 1, day 3, and day 5 after inoculation. The experiment was replicated three times. Error bars indicate the standard deviation of the mean (n = 3). Invisible error bars were smaller than symbols. (b) A representative image of N. europaea grown for 5 days under treatment with MBOA (right flask) and the control (left flask). A turbid culture in the right flask contains grown N. europaea, which was suppressed by MBOA
Next, we refocused on the finding that MBOA incompletely inhibited the bioluminescence of N. europaea (pHLUX20) (Figs. 3 and S6); nevertheless, it strongly suppressed NO2− production from N. europaea without oxidation of NH4+ (Fig. 4). We used recombinant N. europaea (pHLUX20) harboring an expression plasmid vector for bacterial luciferase genes (luxA and luxB) under the transcriptional control of the HAO gene (hao) promoter (Iizumi et al. 1998; Meighen 1993; Sayavedra-Soto et al. 1994). The biochemical basis of the bacterial luciferase, a heterodimeric (αβ) flavin monooxygenase, reaction involves oxidation of a reduced flavin mononucleotide (FMNH2) and a long-chain aliphatic aldehyde (decyl aldehyde in this study) by molecular oxygen to generate oxidized FMN (Campbell et al. 2009; Gibson and Hastings 1962; Meighen and Hastings 1971; Ulitzur and Hastings 1979). Thus, loss of light emission means a decrease of the reducing power in the bacterial cell or destruction of cellular metabolic pathways by nonspecific inhibitors, such as acetone, respiratory inhibitors (NaN3 and Na2S), and heavy metals (HgCl2) (Backhaus and Grimme 1999; Hooper and Terry 1973; Parvez et al. 2006). In addition, inhibition of AMO decreases the reducing power in the cell of N. europaea (pHLUX20), causing the loss of light emission, which is recovered upon addition of hydroxylamine (NH2OH) as a substrate for HAO (Iizumi et al. 1998). Because synthetic allylthiourea targets primarily the AMO pathway and does not affect the luminescence of NH2OH-treated N. europaea (pHLUX20) cells, the electron supply to luciferase via activation of HAO is suggested not to be linked to the presence of an AMO inhibitor that causes a decrease in light emission by blocking the electron flow within the cell (Iizumi et al. 1998). In an additional experiment, we unexpectedly observed that AMO-inhibiting MBOA remarkably increased the intensity of bioluminescence of N. europaea incubated in P medium containing NH2OH as the sole nitrogen source (Fig. S8). Based on these results, we hypothesized that the incomplete inhibition of bioluminescence of N. europaea (pHLUX20) in (NH4)2SO4 medium by MBOA was correlated with HAO activity in the cell, which catalyzed the oxidative conversion of NH2OH to NO2−.
To examine the effect of MBOA on HAO, we incubated N. europaea (pHLUX20) in culture medium containing 20 μM NH2OH as the sole nitrogen source under treatment with MBOA in aerobic conditions. MBOA (5 μM and 50 μM) suppressed NO2− production by more than 80% relative to that of the control after inoculation, whereas NO2− production was not affected by 10 μM allylthiourea (Fig. 6).
Time-course analysis of NO2− production converted from NH2OH by N. europaea (pHLUX20) in the presence of MBOA. A culture broth of 7-day-old N. europaea (pHLUX20) (250 μL, O.D. 660 = 1.0) was inoculated into 100 mL P medium containing 20 μM NH2OH as the sole nitrogen source and MBOA (5 μM and 50 μM) and incubated at 150 rpm and 30 °C. Allylthiourea (AT, 10 μM) and water was used instead of MBOA as the positive control and control, respectively. After the incubation for 0 min (start: inoculated time), 1 h, and 3 h, an aliquot of the broth was collected to measure the NO2− concentration. Error bars indicate the standard deviation of the mean (n = 3). Invisible error bars were smaller than symbols
In contrast to the inhibitory activity of MBOA on both AMO and HAO, the bioluminescence of N. europaea (pHLUX20) in NH2OH medium (Figs. 6 and S8) was enhanced. Given that nonspecific inhibitors (e.g., HgCl2 and Na2S) decrease light emission in N. europaea (pHLUX20) (Iizumi et al. 1998), one possible explanation of this phenomenon is that the reducing power for luciferase was supplied via activation of other metabolic pathways. Further studies on protein–compound interactions using HAO and MBOA are required.
Collectively, these results showed that MBOA inhibited the AMO and HAO pathways, together with cell growth of N. europaea (pHLUX20).
BNI ability of MBOA in soil incubation test
To assess the potency of MBOA against nitrification in the soil, we performed a time-course analysis of NO2− production in a soil incubation test. We incubated soil (10 g, dried weight) in (NH4)2SO4 buffer solution (100 mL) under treatment with MBOA (0.8 mg in 100 mL solution: 50 μM; 1.6 mg in 100 mL solution: 100 μM) and sampled a soil slurry for 7 days after inoculation. We measured the concentrations of NO2− and NO3− in each sample based on the DAN reaction method.
MBOA dose-dependently suppressed NO2− and NO3− production for 4 days of incubation (Fig. 7a and b). The 50 μM and 100 μM MBOA-treated soil incubated for 4 days suppressed NO2− production by 80% and > 99%, respectively (Fig. 7a). In addition, we have confirmed the inhibitory effect of DCD (a commercial nitrification inhibitor) which suppressed the production of NO2− and NO3− in these soil incubation studies (Fig. 7a and b). In contrast, the production of NO2− and NO3− was notably enhanced in MBOA-treated soil after incubation for 5 days (Fig. 7a and b).
Effect of MBOA on nitrification (a and b) and degradation of MBOA in soil (c). MBOA (0.8 mg for 50 μM and 1.6 mg for 100 μM) was added to air-dried soil (10 g) in 750 μM (NH4)2SO4 buffer solution (100 mL). Water was added to the soil solution, instead of MBOA, as the control. An aqueous solution of dicyandiamide (DCD, final concentration: 327 μM) was used as a positive control. Sterilized soil (ST) was used as a negative control. Each soil solution was incubated on a rotary shaker at 100 rpm at 20 °C. An aliquot of the soil solution was collected at 1 h, days 1, 2, 3, 4, 5, and 7 after the start of incubation. All collected solutions were centrifuged, and the supernatant was used for analysis of NO2− and NO3− production and MBOA stability. Error bars indicate the standard deviation of the mean (n = 3). Invisible error bars were smaller than symbols. (c) Stability of MBOA in soil was estimated based on the peak area of MBOA monitored at 287 nm by RP-HPLC. MBOA (50 μM and 100 μM) was maintained in non-sterilized soil for 4 days, but was subsequently not detected owing to microbial biodegradation. MBOA was not degraded in sterilized soil (ST) for 7 days. The difference in initial concentration after addition of MBOA (0.8 mg) between non-sterilized and sterilized soil is attributed to the absorptivity of MBOA by each soil
In parallel with the soil incubation assay, we examined the stability of MBOA in the soil for 7 days. We quantified the peak area of MBOA in MBOA-treated soil by RP-HPLC monitored at 287 nm (Fig. S9). The concentration in the soil of MBOA (50 μM and 100 μM treatments) decreased during incubation for 4 days and was not detected after incubation for 5 days (Figs. 7c and S10). In contrast, MBOA remained detectable in sterilized soil for 7 days (Figs. 7c and S10). Hence, it was strongly suggested that MBOA was biodegraded by soil microorganisms during incubation. In addition, the concentrations of NO2− and NO3− in MBOA-treated soil increased concomitant with MBOA degradation (Fig. 7a–c).
Discussion
Total BNI activity of MBOA in exudates from maize roots
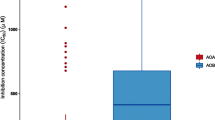
A combination of chromatography guided by a bioluminescence assay enabled us to identify MBOA as the major BNI-active compound in hydrophilic exudates of maize roots (Figs. 1, 2 and 3). To clarify the contribution of MBOA to total BNI activity, we quantified MBOA in fraction M, based on its peak area at 287 nm by HPLC, which was fractionated from hydrophilic exudates (Figs. 1, S9, and S11). The weight of MBOA in fraction M (540 mg) was calculated to be 5.0 mg (Fig. S11). Given that the total BNI activity of hydrophilic exudates (24 g, 18,256 ATU) was concentrated in fraction M containing the BNI-active MBOA (5.0 mg, BNI activity per amount: 0.396 μg/ATU), the total BNI activity of MBOA in hydrophilic exudates was calculated as 12,605 ATU (Fig. 8a). As other BNI compounds have not been identified owing to their weak activities, we elucidated MBOA as the predominant BNI-contributing compound in hydrophilic exudates of maize roots (contributing 69% of the total BNI activity). In our previous study, we have identified HDMBOA (110 mg, 2400 ATU) and zeanone (0.1 mg, 2100 ATU) as the major hydrophobic BNI-active compounds released from maize roots (220 mg, 7700 ATU), together accounting for 62% of the total BNI activity (Fig. 8b) (Otaka et al. 2022). By combining the hydrophilic and hydrophobic exudates, it was revealed that MBOA accounts for nearly 50% of the total BNI activity released from maize roots (Fig. 8c). In turn, HDMBOA and zeanone respectively contributed 9% and 8% to the BNI activities of maize. Hence, we determined that MBOA is the predominant BNI-contributing compound in all exudates of maize roots.
Contributions of identified compounds to BNI activities in root exudates of maize (a–c). (a) MBOA was identified as the most abundant BNI-active compound in hydrophilic exudates. (b) Two major BNI-contributing compounds, HDMBOA and zeanone, were identified in hydrophobic exudates (Otaka et al. 2022). (c) Sum of total BNI activities for hydrophilic and hydrophobic exudates (a + b) indicating that MBOA contributed approximately half of the total BNI activity in root exudates
Although a diverse range of bioactivities among secondary metabolites have been discovered, unlike primary metabolites (i.e., protein, starch, and sugar), secondary metabolites are not considered essential for the growth and development of plants. In addition, the total productivity of our identified BNI secondary metabolites is much smaller than that of root tissue in maize; therefore, the metabolic cost for biosynthesis of BNI compounds from the carbon source in roots may have a negligible impact on the growth of maize.
Collectively, the present results suggested that three BNI-active compounds (MBOA, zeanone, and HDMBOA) are largely responsible for BNI activity released from maize roots, which can be the targets for genetic improvement efforts to improve BNI-capacity in maize.
Occurrence mechanism of MBOA from maize
Although the benzoxazolone MBOA was the most abundant BNI-contributing compound in maize root exudates, the biosynthetic pathway to form the 1,3-benzoxazol-2-one skeleton has not been elucidated. To date, many researchers have investigated benzoxazolones generated by non-enzymatic reactions from more chemically reactive biosynthesized benzoxazinoids (BXs) with a 1,4-benzoxazin-3-one skeleton (Cotton et al. 2019; Klun et al. 1970; Kosemura et al. 1994; Virtanen et al. 1960; Woodward et al. 1978). In particular, MBOA has been reported to be the primary product from three BXs: HDMBOA, 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA), and HMBOA (Fig. S1), which have N-methoxy, N-OH, and N-H groups, respectively (Atkinson et al. 1991; Grambow et al. 1986). HDMBOA has been reported to be the dominant BX in organic exudates of maize seedlings (Otaka et al. 2022; Zhang et al. 2000). The half-life of HDMBOA in aqueous solution is much shorter than that of the other two BXs because the N-methoxy group is less stable than the N-OH and N-H groups, which we also confirmed by auto-conversion of HDMBOA into MBOA in water (Fig. S12) (Grambow et al. 1986; Maresh et al. 2006). One intermediate in the conversion of HDMBOA to MBOA, o-imidoquinone linked to formic acid and MeOH, has been established (Fig. S13) (Grambow et al. 1986; Maresh et al. 2006). Importantly, we also observed that HDMBOA was converted to MBOA whether in sterilized or non-sterilized soil within 24 h (Fig. 9a), strongly suggesting that this phenomenon does not involve biological conversion in soil.
Time-course analyses of conversion of HDMBOA (a) and HDMBOA-β-glucoside (b) to MBOA in soil and the generation mechanism of MBOA from HDMBOA-β-glucoside (c). (a and b) To 5 μL solution of HDMBOA (350 μM) in acetone or HDMBOA-β-glucoside (350 μM) in MeOH was added wet soil (100 mg in 500 μL water). The mixture in a glass vial was incubated at 25 °C for 24 h. Sterilized soil was used as a negative control. After each sample was centrifuged, the supernatant of each collected sample (5 μL) was analyzed using HPLC with monitoring at 210 nm [1% acetonitrile/99% H2O (0.1% formic acid) (start) to 30% acetonitrile/70% H2O (0.1% formic acid) (13 min), 0.4 mL min−1]. (a and c) HDMBOA was non-biologically converted into MBOA in soil after 24 h. (b and c) HDMBOA-β-glucoside was irreversibly hydrolyzed to MBOA in soil. In sterilized soil, the peak of HDMBOA-β-glucoside was retained
As an internal compound in maize roots, HDMBOA is stored not only as a stand-alone compound (agricone form) but also in a glycosylated form (HDMBOA-β-glucoside), which can be enzymatically hydrolyzed into agricone (HDMBOA) and glucose (Ahmad et al. 2011; Niemeyer 2009; Otaka et al. 2022). We have previously observed that both β-amylase and β-glucosidase, which are generally present in higher plants and microbes, catalyze the hydrolysis of HDMBOA-β-glucoside to give HDMBOA and MBOA (Gu et al. 2006; Lauriere et al. 1992; Otaka et al. 2022). Based on these results, we hypothesize that HDMBOA-β-glucoside can be hydrolyzed by maize roots and/or soil microbes. In maize roots, hydrolysis of HDMBOA-β-glucoside is performed by a metabolic process in the root tissue, such as turnover of cytoplasmic material, detachment of the root cap, and root elongation (Brzobohaty et al. 1993; McLoughlin et al. 2018). However, soil microbes can also hydrolyze HDMBOA-β-glucoside. Indeed, we observed that HDMBOA-β-glucoside was irreversibly converted into MBOA in non-sterilized soil, but this biological reaction was not observed in sterilized soil (Fig. 9b). HDMBOA generated via biological hydrolysis is then non-biologically converted into MBOA, as shown in Fig. 9c.
Taken together, we suggest that part of the released MBOA from maize roots can also come from transformations of BXs accumulated both on the surface and within roots. In particular, HDMBOA and HDMBOA-β-glucoside are suggested to be the crucial precursors of MBOA.
Plausible BNI mechanism in maize
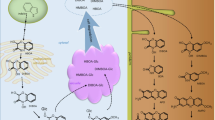
On the basis of the collective results, we propose a comprehensive BNI mechanism for maize (Fig. 10) (Otaka et al. 2022). Maize releases three compounds responsible for BNI activity from the roots: MBOA (ED50 = 0.76 μM) in the hydrophilic portion, and zeanone (ED50 = 2 μM) and HDMBOA (ED50 = 13 μM) in the hydrophobic portion (Figs. 2 and S1). MBOA has the ability to suppress nitrification in the soil, in which it can block at least the AMO and HAO pathways in N. europaea (Figs. 4, 5, 6 and 7). BNI activity attributable to MBOA can be diffused from the rhizosphere because of its water-solubility (0.56 mg/mL at 25 °C), whereas the BNI-effective area of naturally occurring zeanone and HDMBOA may be limited in the rhizosphere owing to their low affinity with water.
Proposed BNI mechanism in maize. Maize can secrete three BNI-active compounds from the roots: zeanone and HDMBOA in hydrophobic exudates, and MBOA in hydrophilic exudates. Abundant HDMBOA is non-biologically converted into MBOA after secretion into the rhizosphere. HDMBOA-β-glucoside (within the roots), when released, is transformed into MBOA via biological hydrolysis and following auto-conversion of HDMBOA in the soil
Released HDMBOA is eventually converted into the more stable MBOA via a non-biological process (Fig. 9a and c). When conversion of 110 mg HDMBOA (0.488 mmol) in hydrophobic exudates (220 mg) gives rise to MBOA (80.6 mg, 0.488 mmol), the total BNI activity caused by MBOA (12,605 ATU) is potentially increased to 351,539 ATU (Hiradate 2006). It has been reported that the basic condition (>pH 7.0, in phosphate buffer) favors the rapid conversion from HDMBOA to MBOA (Maresh et al. 2006). In addition, acidification of the maize rhizosphere has been reported when maize takes up NH3 by the release of H+ from root cells (Gollany and Schumacher 1993; Jing et al. 2010; Zhou et al. 2009). Therefore, it is crucial to consider that the yield of MBOA from HDMBOA is regulated by the physicochemical conditions in the rhizosphere (e.g., pH, water content, temperature, and electric charge). In addition to HDMBOA, HDMBOA-β-glucoside released from within the roots is also eventually transformed to MBOA via biological hydrolysis and following non-biological conversion of HDMBOA, which enters the rhizosphere together with water to exhibit BNI activity (Fig. 9b and c).
Although MBOA is much more stable than BXs (HDMBOA and HDMBOA-β-glucoside) in a soil environment, it can be biochemically degraded to downstream products by soil microorganisms that weaken the BNI activity (Figs. 7c and S10) (Friebe et al. 1998; Macias et al. 2009). However, in parallel with the degradation of MBOA, MBOA may be continuously supplied as long as maize can release BXs into the rhizosphere. Hence, the longevity of BNI activity derived from MBOA is suggested to be sustained in the soil, which is an advantage for maize. Therefore, we attributed MBOA to be the key component of BNI in maize (Fig. 10).
Conclusion
We have established that MBOA as the major hydrophilic BNI-active compound released from maize roots. MBOA suppressed the conversion not only of NH3 to NO2− (AMO pathway) but also NH2OH to NO2− (HAO pathway) in N. europaea. In a soil incubation test, over 80% of NO2− production was suppressed by 50 μM MBOA; however, it’s soil-residence time is only 5 days and degraded subsequently from soil microbial activity. Quantification experiments revealed that MBOA contributed nearly to 50% of the BNI activity released from maize roots. Two BXs, HDMBOA and HDMBOA-β-glucoside (which are chemically and biologically unstable in soil), are converted to a more stable BNI-active MBOA in the soil (through a soil-chemical process). Overall, we have shown that MBOA is a key component in the BNI activity of maize.
Data availability
The datasets generated during the current study are available from JIRCAS on reasonable request.
References
Ahmad S et al (2011) Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol 157:317–327. https://doi.org/10.1104/pp.111.180224
Anai T, Aizawa H, Ohtake N, Kosemura S, Yamamura S, Hasegawa K (1996) A new auxin-inhibiting substance, 4-Cl-6,7-dimethoxy-2-benzoxazolinone, from light-grown maize shoots. Phytochemistry 42:273–275. https://doi.org/10.1016/0031-9422(95)00986-8
Atkinson J, Morand P, Arnason JT, Niemeyer HM, Bravo HR (1991) Analogs of the cyclic hydroxamic acid 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one (DIMBOA): decomposition to benzoxazolinones and reaction with β-mercaptoethanol. J Org Chem 56:1788–1800. https://doi.org/10.1021/jo00005a025
Backhaus T, Grimme LH (1999) The toxicity of antibiotic agents to the luminescent bacterium Vibrio fischeri. Chemosphere 38:3291–3301. https://doi.org/10.1016/s0045-6535(98)00560-8
Brzobohaty B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K (1993) Release of active cytokinin by a β-glucosidase localized to the maize root meristem. Science 262:1051–1054. https://doi.org/10.1126/science.8235622
Campbell ZT, Weichsel A, Montfort WR, Baldwin TO (2009) Crystal structure of the bacterial luciferase/flavin complex provides insight into the function of the β subunit. Biochemistry 48:6085–6094. https://doi.org/10.1021/bi900003t
Chang SW, Hyman MR, Williamson KJ (2002) Cooxidation of naphthalene and other polycyclic aromatic hydrocarbons by the nitrifying bacterium, Nitrosomonas europaea. Biodegradation 13:373–381. https://doi.org/10.1023/a:1022811430030
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017) Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat Plants 3:17074. https://doi.org/10.1038/nplants.2017.74
Cotton TEA, Petriacq P, Cameron DD, Meselmani MA, Schwarzenbacher R, Rolfe SA, Ton J (2019) Metabolic regulation of the maize rhizobiome by benzoxazinoids. ISME J 13:1647–1658. https://doi.org/10.1038/s41396-019-0375-2
Friebe A, Vilich VV, Hennig L, Kluge M, Sicker D (1998) Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and Fusarium culmorum. Appl Environ Microbiol 64:2386–2391. https://doi.org/10.1128/AEM.64.7.2386-2391.1998
Ghatak A, Chaturvedi P, Waldherr S, Subbarao GV, Weckwerth W (2022) PANOMICS at the interface of root-soil microbiome and BNI. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2022.08.016
Gibson QH, Hastings JW (1962) The oxidation of reduced flavin mononucleotide by molecular oxygen. Biochem J 83:368–377. https://doi.org/10.1042/bj0830368
Gollany HT, Schumacher TE (1993) Combined use of colorimetric and microelectrode methods for evaluating rhizosphere Ph. Plant Soil 154:151–159. https://doi.org/10.1007/Bf00012520
Grambow HJ, Lückge J, Klausener A, Müller E (1986) Occurrence of 2-(2-hydroxy-4,7-dimethoxy-2H-1,4-benzoxazin-3-one)-β-D-glucopyranoside in Triticum aestivum leaves and its conversion into 6-methoxy-benzoxazolinone. Z Naturforsch C 41:684–690. https://doi.org/10.1515/znc-1986-7-804
Gu R et al (2006) Isolation of a maize β-glucosidase gene promoter and characterization of its activity in transgenic tobacco. Plant Cell Rep 25:1157–1165. https://doi.org/10.1007/s00299-006-0177-7
Hasegawa K, Togo S, Urashima M, Mizutani J, Kosemura S, Yamamura S (1992) An auxin-inhibiting substance from light-grown maize shoots. Phytochemistry 31:3673–3676. https://doi.org/10.1016/s0031-9422(00)97506-x
Hiradate S (2006) Isolation strategies for finding bioactive compound: specific activity vs. total activity. Natur Prod Pest Manag 927:113–126
Hooper AB, Terry KR (1973) Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol 115:480–485. https://doi.org/10.1128/jb.115.2.480-485.1973
Iizumi T, Mizumoto M, Nakamura K (1998) A bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Appl Environ Microbiol 64:3656–3662. https://doi.org/10.1128/AEM.64.10.3656-3662.1998
Ivancic I, Degobbis D (1984) An optimal manual procedure for ammonia analysis in natural-waters by the indophenol blue method. Water Res 18:1143–1147. https://doi.org/10.1016/0043-1354(84)90230-6
Jing J, Rui Y, Zhang F, Rengel Z, Shen J (2010) Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res 119:355–364. https://doi.org/10.1016/j.fcr.2010.08.005
Juliette LY, Hyman MR, Arp DJ (1993) Inhibition of ammonia oxidation in Nitrosomonas europaea by sulfur compounds: thioethers are oxidized to sulfoxides by ammonia monooxygenase. Appl Environ Microbiol 59:3718–3727. https://doi.org/10.1128/aem.59.11.3718-3727.1993
Keener WK, Arp DJ (1994) Transformations of aromatic compounds by Nitrosomonas europaea. Appl Environ Microbiol 60:1914–1920. https://doi.org/10.1128/aem.60.6.1914-1920.1994
Klun JA, Tipton CL, Robinson JF, Ostrem DL, Beroza M (1970) Isolation and identification of 6,7-dimethoxy-2-benzoxazolinone from dried tissues of Zea-Mays (L) and evidence of its cyclic hydroxamic acid precursor. J Agr Food Chem 18:663–665. https://doi.org/10.1021/jf60170a017
Kosemura S, Yamamura S, Anai T, Hasegawa K (1994) Chemical studies on 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one in connection with 6-methoxy-2-benzoxazolinone, an auxin-inhibiting substance of Zea mays L. Tetrahedron Lett 35:8221–8224. https://doi.org/10.1016/0040-4039(94)88287-8
Kosemura S, Emori H, Yamamura S, Anai T, Aizawa H, Ohtake N, Hasegawa K (1995) Isolation and characterization of 4-chloro-6,7-dimethoxybenzoxazolin-2-one, a new auxin-inhibiting benzoxazolinone from Zea mays. Chem Lett 24:1053–1054. https://doi.org/10.1246/cl.1995.1053
Koyama T (1955) Studies on the constituents of Coix species. II. Yakugaku Zasshi 75:702–704. https://doi.org/10.1248/yakushi1947.75.6_702
Koyama T, Yamato M (1955) Studies on the constituents of Coix species. I. Yakugaku Zasshi 75:699–701. https://doi.org/10.1248/yakushi1947.75.6_699
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276. https://doi.org/10.1038/nrmicro.2018.9
Lauriere C, Doyen C, Thevenot C, Daussant J (1992) β-Amylases in cereals: a study of the maize β-amylase system. Plant Physiol 100:887–893. https://doi.org/10.1104/pp.100.2.887
Lewis RF, Pramer D (1958) Isolation of Nitrosomonas in pure culture. J Bacteriol 76:524–528. https://doi.org/10.1128/jb.76.5.524-528.1958
Macias FA, Marin D, Oliveros-Bastidas A, Molinillo JM (2009) Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Nat Prod Rep 26:478–489. https://doi.org/10.1039/b700682a
Maresh J, Zhang J, Lynn DG (2006) The innate immunity of maize and the dynamic chemical strategies regulating two-component signal transduction in agrobacterium tumefaciens. ACS Chem Biol 1:165–175. https://doi.org/10.1021/cb600051w
McLoughlin F, Augustine RC, Marshall RS, Li F, Kirkpatrick LD, Otegui MS, Vierstra RD (2018) Maize multi-omics reveal roles for autophagic recycling in proteome remodelling and lipid turnover. Nat Plants 4:1056–1070. https://doi.org/10.1038/s41477-018-0299-2
Meighen EA (1993) Bacterial bioluminescence: organization, regulation, and application of the lux genes. FASEB J 7:1016–1022. https://doi.org/10.1096/fasebj.7.11.8370470
Meighen EA, Hastings JW (1971) Binding site determination from kinetic data. J Biol Chem 246:7666–7674. https://doi.org/10.1016/s0021-9258(19)45827-4
Nagao T, Otsuka H, Kohda H, Sato T, Yamasaki K (1985) Benzoxazinones from Coix lachryma-Jobi var. Ma-Yuen. Phytochemistry 24:2959–2962. https://doi.org/10.1016/0031-9422(85)80035-2
Niemeyer HM (2009) Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: key defense chemicals of cereals. J Agric Food Chem 57:1677–1696. https://doi.org/10.1021/jf8034034
Nussler AK, Glanemann M, Schirmeier A, Liu L, Nussler NC (2006) Fluorometric measurement of nitrite/nitrate by 2,3-diaminonaphthalene. Nat Protoc 1:2223–2226. https://doi.org/10.1038/nprot.2006.341
Otaka J, Subbarao GV, Ono H, Yoshihashi T (2022) Biological nitrification inhibition in maize-isolation and identification of hydrophobic inhibitors from root exudates. Biol Fertil Soils 58:251–264. https://doi.org/10.1007/s00374-021-01577-x
Parvez S, Venkataraman C, Mukherji S (2006) A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ Int 32:265–268. https://doi.org/10.1016/j.envint.2005.08.022
Prosser JI (1990) Autotrophic nitrification in bacteria. Adv Microb Physiol 30:125–181. https://doi.org/10.1016/S0065-2911(08)60112-5
Rivett MO, Buss SR, Morgan P, Smith JW, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42:4215–4232. https://doi.org/10.1016/j.watres.2008.07.020
Sayavedra-Soto LA, Hommes NG, Arp DJ (1994) Characterization of the gene encoding hydroxylamine oxidoreductase in Nitrosomonas europaea. J Bacteriol 176:504–510. https://doi.org/10.1128/jb.176.2.504-510.1994
Scheer C, Fuchs K, Pelster DE, Butterbach-Bahl K (2020) Estimating global terrestrial denitrification from measured N2O:(N2O + N2) product ratios. Curr Opin Environ Sustain 47:72–80. https://doi.org/10.1016/j.cosust.2020.07.005
Smissman EE, LaPidus JB, Beck SD (1957) Isolation and synthesis of an insect resistance factor from corn plants. J Am Chem Soc 79:4697–4698. https://doi.org/10.1021/ja01574a032
Stayner LT, Almberg K, Jones R, Graber J, Pedersen M, Turyk M (2017) Atrazine and nitrate in drinking water and the risk of preterm delivery and low birth weight in four Midwestern states. Environ Res 152:294–303. https://doi.org/10.1016/j.envres.2016.10.022
Subbarao GV, Searchinger TD (2021) Opinion: a "more ammonium solution" to mitigate nitrogen pollution and boost crop yields. Proc Natl Acad Sci U S A 118. https://doi.org/10.1073/pnas.2107576118
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006) A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112. https://doi.org/10.1007/s11104-006-9094-3
Subbarao GV et al (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Natl Acad Sci U S A 106:17302–17307. https://doi.org/10.1073/pnas.0903694106
Subbarao GV et al (2013a) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259. https://doi.org/10.1007/s11104-012-1419-9
Subbarao GV et al (2013b) A paradigm shift towards low-nitrifying production systems: the role of biological nitrification inhibition (BNI). Ann Bot 112:297–316. https://doi.org/10.1093/aob/mcs230
Subbarao GV et al (2021) Enlisting wild grass genes to combat nitrification in wheat farming: a nature-based solution. Proc Natl Acad Sci U S A 118. https://doi.org/10.1073/pnas.2106595118
Sun L, Lu Y, Yu F, Kronzucker HJ, Shi W (2016) Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol 212:646–656. https://doi.org/10.1111/nph.14057
Thakur IS, Medhi K (2019) Nitrification and denitrification processes for mitigation of nitrous oxide from waste water treatment plants for biovalorization: challenges and opportunities. Bioresour Technol 282:502–513. https://doi.org/10.1016/j.biortech.2019.03.069
Tian H et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256. https://doi.org/10.1038/s41586-020-2780-0
Tsikas D (2007) Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci 851:51–70. https://doi.org/10.1016/j.jchromb.2006.07.054
Ulitzur S, Hastings JW (1979) Evidence for tetradecanal as the natural aldehyde in bacterial bioluminescence. Proc Natl Acad Sci U S A 76:265–267. https://doi.org/10.1073/pnas.76.1.265
Vazquez E, Teutscherova N, Dannenmann M, Tochterle P, Butterbach-Bahl K, Pulleman M, Arango J (2020) Gross nitrogen transformations in tropical pasture soils as affected by Urochloa genotypes differing in biological nitrification inhibition (BNI) capacity. Soil Biol Biochem 151:108058. https://doi.org/10.1016/j.soilbio.2020.108058
Venis MA, Watson PJ (1978) Naturally occurring modifiers of auxin-receptor interaction in corn: identification as benzoxazolinones. Planta 142:103–107. https://doi.org/10.1007/Bf00385127
Virtanen AI, Hietala PK, Wahlroos O (1957) Antimicrobial substances in cereals and fodder plants. Arch Biochem Biophys 69:486–500. https://doi.org/10.1016/0003-9861(57)90513-1
Virtanen AI, Hietala PK, Norén B, Levitin NE, Westin G (1960) Precursors of benzoxazolinone in rye plants. I. Precursor II, the aglucone. Acta Chem Scand 14:499–502. https://doi.org/10.3891/acta.chem.scand.14-0499
Wendeborn S (2020) The chemistry, biology, and modulation of ammonium nitrification in soil. Angew Chem Int Ed Engl 59:2182–2202. https://doi.org/10.1002/anie.201903014
Woodward MD, Corcuera LJ, Helgeson JP, Upper CD (1978) Decomposition of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one in aqueous solutions. Plant Physiol 61:796–802. https://doi.org/10.1104/pp.61.5.796
Zakir HA et al (2008) Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (sorghum bicolor). New Phytol 180:442–451. https://doi.org/10.1111/j.1469-8137.2008.02576.x
Zhang J, Boone L, Kocz R, Zhang C, Binns AN, Lynn DG (2000) At the maize/agrobacterium interface: natural factors limiting host transformation. Chem Biol 7:611–621. https://doi.org/10.1016/s1074-5521(00)00007-7
Zhou LL, Cao J, Zhang FS, Li L (2009) Rhizosphere acidification of faba bean, soybean and maize. Sci Total Environ 407:4356–4362. https://doi.org/10.1016/j.scitotenv.2009.02.006
Acknowledgements
We thank Ms. Yukiko Ishikawa and Ms. Sanae Suzuki for cultivation of N. europaea, and Ms. Hiroko Aoki, Ms. Yoko Koizumi, Ms. Masami Aoyama, Mr. Makoto Yamamoto, Dr. Gao Xiang, and Mr. Raphael Obias Mubanga for sampling of maize plants at JIRCAS. We thank Dr. Kazuhiro Nakahara at JIRCAS for fruitful discussions. We are grateful to Dr. Ikuko Maeda at the NARO for NMR measurements. We thank Robert McKenzie, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Funding
This work was supported by the research project of JIRCAS entitled: “Development of ecologically sustainable agricultural systems through practical use of the BNI function”.
Author information
Authors and Affiliations
Contributions
Junnosuke Otaka, Guntur Venkata Subbarao, and Tadashi Yoshihashi designed the experiments. Junnosuke Otaka performed chemical experiments, including structural elucidation of compounds. Junnosuke Otaka, Guntur Venkata Subbarao and Jiang MingLi performed bioassays using N. europaea. Junnosuke Otaka and Jiang MingLi performed the soil incubation experiment. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Devrim Coskun.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 4977 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otaka, J., Subbarao, G.V., MingLi, J. et al. Isolation and characterization of the hydrophilic BNI compound, 6-methoxy-2(3H)-benzoxazolone (MBOA), from maize roots. Plant Soil 489, 341–359 (2023). https://doi.org/10.1007/s11104-023-06021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06021-7