Abstract
Aims
While it is well-established that nitrogen (N) availability regulates elevated [CO2] (eCO2) effects on plant growth and soil carbon (C) storage in N-limited environments, there are fewer studies investigating the role of phosphorous (P) supply on such responses in P-limited environments. In this study, we explored whether P fertilization influences the response of plant growth, soil enzyme activity and C fluxes to eCO2, and determined how different levels of water availability regulate these processes.
Methods
We used soil collected from a temperate, P-limited Eucalyptus woodland containing the native soil seed bank to grow a potted replica of local understory communities. We exposed the emerging communities to eCO2 under two contrasting water levels and two levels of P fertilization. We assessed plant biomass allocation, the rhizosphere activity of extracellular enzymes related to C, N and P cycles, and pot-level CO2 fluxes.
Results
The positive effects of eCO2 on plant production and ecosystem C dynamics were strongly constrained by low levels of P availability. Enhanced water supply increased rhizosphere enzyme activity with minor impacts on plant biomass responses to eCO2. Our data also suggest that plant and microbial mechanisms that increase nutrient release from SOM may not be able to overcome this P limitation.
Conclusions
While current Earth System Models predict positive feedback responses of terrestrial ecosystems on C storage under eCO2, here we emphasize the importance of accounting for the widespread phenomenon of P-limitation in such responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A primary scientific question in climate change research is the extent to which terrestrial ecosystems will continue strengthening their role as net CO2 sinks under rising atmospheric [CO2] concentrations (Körner et al. 2007). Elevated CO2 (eCO2) has been shown to stimulate plant carbon uptake in a wide range of plant species and experimental conditions (Poorter et al. 2012). However, plant acclimation (Crous et al. 2011) and/or concomitant increases in soil respiration may counteract such ecosystem-scale increases in the long term (Jiang et al. 2020). While organ-level responses to eCO2 are relatively well-known, particularly for leaves (Leakey et al. 2009), scaling-up such responses to the whole plant or community-level is challenging due to variability in the physiological effects of eCO2 among different species and organs (i.e., shade vs. sun leaves or absorptive vs. transport roots; Laliberté 2017). In addition, whole-system approaches under natural conditions are scarce due to the high costs associated with large-scale experimental set-ups (Filion et al. 2000), which also limits their replicability across ecosystem types and across gradients of resource availability (Norby et al. 2016). However, experimental approaches that can account for the myriad biotic and abiotic interactions occurring in natural conditions, while allowing for the simultaneous manipulation of other environmental drivers such as soil resources (e.g., water and nutrients) that are known to modulate such responses, are needed to make reliable predictions about the future impacts of climate change.
Traditionally, realistic predictions of eCO2 effects have relied on Free Air CO2 Enrichment (FACE) experiments, mostly conducted in temperate regions and predominantly in N and/or water-limited stands within the Northern Hemisphere (Morgan et al. 2004; Norby and Zak 2011). However, there is still limited evidence for effects of eCO2 on ecosystem functioning in the highly weathered, low-phosphorus (P) tropical and subtropical ecosystems from the Southern Hemisphere (Norby et al. 2016).In these ecosystems, P frequently limits the growth of plants, with the majority of soil P associated with insoluble compounds that are not readily available for biological uptake (Dijsktra et al. 2013). For these, the question of whether eCO2 can stimulate soil P availability and/or increase its uptake by plants, as has been seen for nitrogen (N) in N-limited stands, has received comparatively little attention (Leuzinger and Hättenschwiler 2013). In naturally N-limited stands, increased plant transfer of C to the rhizosphere – in the form of labile C-rich compounds from fine root exudates and litter – has been associated with greater soil microbial enzyme activity under eCO2 (Kuzyakov 2010; Phillips et al. 2011) and this, in turn, regulates both plant productivity and ecosystem CO2 flux under eCO2 (Finzi et al. 2015). However, whether enhanced exudation of labile C compounds under eCO affects enzymes in naturally P-deficient soils is still unclear (Dijkstra et al. 2013; Piñeiro et al. 2022). This is because P, in contrast to N, can be mobilized directly by fine roots from both organic and inorganic sources through abiotic reactions (i.e. dissolution/desorption and hydrolysis processes, respectively; Richardson et al. 2009).
Along with plant adaptations to mine for nutrients under eCO2, one must take into account the direct effects of eCO2 on plant water relations and its feedbacks on water and nutrient availability in the soil (Wullschleger et al. 2002). Along with increased rates of photosynthesis, eCO2 often decreases stomatal conductance, reducing transpirational water loss (Leakey et al. 2009) and consequently resulting in increases in both water use efficiency (WUE) and soil moisture (Morgan et al. 2004; Leuzinger and Hättenschwiler 2013). These eCO2-associated effects on plant water use can ameliorate the negative effects of drought on growth, particularly in semi-arid ecosystems (Morgan et al. 2004). In addition, soil water savings may have positive effects on nutrient availability because soil moisture and nutrient mineralization rates are often positively correlated (Leuzinger and Hättenschwiler 2013). Whilst many studies have looked at interactions between eCO2 and drought, fewer studies have considered interactions with enhanced levels of water supply (Wullschleger et al. 2002). Understanding this is important because when soil water is relatively high, positive feedbacks of soil water savings may be negligible. Furthermore, with climate predictions of greater frequency of large, deluge rainfall events (Webb & Hennessy, 2015), understanding how eCO2 interacts with greater than average levels of rainfall and soil water availability is highly relevant.
In this study, we used soil collected from a temperate, P-limited eucalypt woodland (Crous et al. 2015) located in Eastern Australia containing the native soil seed bank, to grow a potted replica of local understory communities. In these ecosystems, understory plant communities greatly contribute to ecosystem diversity and functioning via its effects on tree seedling emergence, and biogeochemical processes (Kolari et al. 2006; Landuyt et al. 2019). This approach allows investigation of the main processes regulating whole-ecosystem functions while maintaining biotic and abiotic interactions between plant-soil-microbial assemblages, and focuses on an often overlooked portion of woodland vegetation. We investigated how the availability of two key soil resources (i.e. P [no P added; + 30 mg P kg soil−1] and water affect the responses of the emergent understory plant community and ecosystem functioning to eCO2, along with the feedbacks of key mechanisms underpinning ecosystem carbon exchange. In particular, we hypothesize that (H1) total plant biomass production will be greatest under higher levels of CO2, water and P supply (Körner et al. 2007; Piñeiro et al. 2017). In addition, we expect (H1a) positive interactions between P addition and eCO2 on plant production as alleviation of P constraints on productivity will allow the benefits of CO2 fertilisation to be realised. In contrast (H1b), we expect stronger positive effects of P addition than of water addition under eCO2 due to the particularly strong P-limitation of these soils (Crous et al. 2015). We also predict that (H2) eCO2 will increase fine root production and rhizosphere enzyme activity under low P and water supply conditions as a functional response to greater nutrient demand (Piñeiro et al. 2020), but not under high levels of these resources. Finally, we hypothesize that (H3) eCO2 will lead to a greater net CO2 uptake rate (i.e. more negative NEE values), although we expect this effect to be bigger under higher water and P supply due to both positive interactions between eCO2 and soil resource availability on biomass production and a lack of stimulation of rhizosphere microbial activity under high-resource conditions. As such, we expect that the balance between GPP and respiration (i.e. NEE) to be more negative under eCO2 than aCO2, particularly when P and water are supplied.
Materials and methods
Experimental setup
The Cumberland Plain woodland that served as the source for soil and its associated seedbank is located near Western Sydney University, at Richmond, NSW, Australia (33°36′39″S, 150°44′23″E). The stand is a P-limited woodland (Crous et al. 2015) dominated by Red Gum (Eucalyptus tereticornis) with a diverse understory community comprised of approximately 70 species, including shrubs such as Breynia oblongifolia and Sida rhombifolia and C3 and C4 grasses such as Microlaena stipoides and Eragrostis curvula as dominant species, respectively (Hasegawa et al. 2018). Mean annual precipitation at this site is ~ 800 mm. Soils, which are part of the Clarendon Formation, have a pH of 5.7, 10 g kg−1 organic C, 1.25 g kg−1 organic N, and 0.062 g kg−1 total P. In April 2015, surface soil (0-20 cm) was collected and split into two separate layers; an organic layer (0–2 cm) containing the native seed bank and an inorganic layer (2–20 cm). Before use, the organic soil (seed source) was homogenized by sieving through a 4 mm sieve and stored in a dark room at 4 °C for cold stratification for a period of two weeks. Inorganic soil was homogenized by sieving through a 4 mm sieve, and air-dried. Three hundred and fifty grams of organic soil were placed on top of 3,300 g of inorganic soil in 64 pots (21 cm. in diameter, 16 cm in height). The bottom of each pot consisted of a PVC cap with four drilled drainage holes covered with 2 mm mesh. The plant community was allowed to naturally develop from the native seed bank, with 12 h of light (approximately 800 µmol m−2 s−1), at 26 °C when lights were on and 21 °C when lights were off, and 40% relative air humidity. Artificial lighting (1100 W lights, P.L. Artificial light Systems, Beamsville, ON) was used whenever the natural light intensity fell below 200 W m2 between 7 A.M. and 6 P.M. (Argus Control Systems Ltd., White Rock, BC). This approach allowed for the development of a realistic plant community as it used the existing soil seed bank and maintained natural soil-microbe-plant interactions, whilst allowing community development to be monitored in a controlled environment (Wurth et al. 1998; Ochoa-Hueso & Manrique 2014).
The experimental design comprised a factorial combination of two levels of CO2 (400 ppm and 570 ppm) and four levels of soil resource supply: P fertilization (0 or 30 kg P ha-1) and its combination with two levels of water supply (50% and 90% of the soil water holding capacity). Four replicate pots for each combination of P and water were placed in each chamber (two chambers under eCO2; 16 pots per chamber) (two chambers under ambient CO2; 16 pots per chamber). In order to minimize chamber effects, pots were randomly swapped between chambers every week throughout the 14 weeks of the experiment. Pots in the high P treatment were amended with 30 kg P ha−1 using commercial triple super phosphate (Ca(H2PO4)2H2O) mixed in with the mineral soil during pot preparation. Phosphorus levels were selected based on previous experiments at the study site (Crous et al. 2015), which showed that similar addition rates increased vegetation growth, and it is line with previous studies on Australian ecosystems that have shown a consistent stimulation of productivity in grasses and annual plants with the addition of 20 to 40 kg P ha−1 (Daws et al. 2013). During the first two weeks of the experiment, all pots were watered to gravimetric soil moisture contents (gSMC) of 21% (i.e., ∽90% of soil water holding capacity) to ensure homogeneous seed germination across water treatments, after which pots were held at treatment targets for soil water content (12% and 20%). The lower moisture level used in our experimental set up (∽60% WHC) serves as reference as it falls within the range for the experimental site during the growing season (Piñeiro et al. 2020). Our higher moisture level (∽90% WHC) was selected to provide enough contrast with reference soil moisture levels. In addition, the EucFACE site, where the soil was collected, often experiences large, deluge rainfall events during the growing season, which results in large periods of high soil moisture (Piñeiro et al. 2020). All pots were weighed and watered to their target weights (to achieve required gSMC) three times per week. This design resulted in eight replicates for each level of CO2, phosphorus and water supply.
Assessment of net ecosystem CO2 exchange
Pot-level CO2 fluxes were measured four times during the experiment (10th June, 2nd July, 11th August and 29th August 2015) using a translucent PVC chamber, 30 cm diameter × 100 cm high, fitted with a circulating fan to ensure good mixing of air within the chamber, and an open-path LI-7500 infrared gas analyzer (LI-COR) for measuring [CO2] (Bachman et al. 2010). Four pots for each treatment combination were placed in custom-fit 30 cm diameter PVC rings and the PVC chamber was sealed on to these. Once sealed, CO2 concentrations were measured for 120 s of stable flux measurements, as a measure of net ecosystem carbon exchange (NEE). After each individual NEE flux measurement was complete, we covered the PVC chamber with black material to block out light (i.e., stop CO2 assimilation) and recorded CO2 concentrations for an additional 120 s to obtain a measure of pot-level respiration (Reco). The chamber was not ventilated between measurements. All CO2 flux measurements were carried out on the same day between 8 am and 12 pm, during stable weather conditions. Carbon dioxide flux (F) was calculated using the following equation:
where dC’/dt is the rate of change in the dry-air molar fraction of C over 120 s, P is the air pressure (kPa) recorded on the LI-7500 internal pressure transducer, Wi is the initial water vapour molar fraction, V is the chamber volume (m3), T is the temperature (Kelvin) and A is the ground area covered by the chamber (m2). GPP was calculated from the relationship: NEE = GPP –Reco. and water use efficiency (WUE) was calculated as GPP/ET (where ET is evapotranspiration). We adopted the standard sign convention for NEE used for eddy covariance, with negative values indicating CO2 uptake by the ecosystem and positive values indicating flux to the atmosphere. Similarly, we used the same convention for WUE, with negative values indicating greater CO2 uptake per unit of water transpired (Baldocchi 2003).
Plant harvest and calculations
All plants were harvested 14 weeks after the experiment commenced, and separated into leaves, stems and roots. Plant species identity and functional group (i.e. C3 or C4 photosynthetic pathway) were recorded at harvest time. Aboveground plant material in each pot was oven dried (70 °C for 48 h) and weighed. Root systems were carefully washed of soil particles, oven dried (70 °C for 48 h) and weighed to determine total root biomass and the proportion of biomass allocated belowground relative to aboveground. Dried leaves from each pot were ground separately and analysed for C and N concentrations with a CHN elemental analyser (LECO TruSpec, LECO Corporation, St Joseph,MI,USA).
Rhizosphere extracellular enzyme activity measurements
Rhizosphere soil samples were obtained by collecting soil particles attached to the finest roots (average diameter < 0.1 mm) sampled from 0–10 cm depth in each pot. The potential activity of seven extracellular enzymes related to C, N and P cycles in rhizosphere soils were assessed fluorometrically following the methods described in Bell et al. (2013). Briefly, we assayed: α-1,4-glucosidase (AG; starch degradation), β-1,4-glucosidase (BG; starch degradation), β-xylosidase (XYL; hemicellulose degradation) and β-D-cellobiohydrolase (CBH; cellulose degradation) for the C cycle; β-1,4-N-acetylglucosaminidase (NAG; chitin degradation) and L-leucine aminopeptidase (LAP; protein degradation) for the N cycle; phosphatase (PHOS; phosphorus mineralization) for the P cycle. Assays were conducted by homogenizing 1 g of soil in 33 ml of 50 mM sodium acetate buffer (pH 5.5) for 1 min. The homogenised solutions along with fluorometric substrates were then added to a 96-deep-well (2 ml) microplate and incubated for 1.5 h at 35 °C. Standard curves were prepared for each sample by adding 800 µL of soil slurry to 200 µL of standard stocks (4-methylumbellfferone (MUB) of 7-amino-4-methylcoumarin (MUC) at a range of different concentrations. Following incubation, the supernatant solution was transferred into corresponding wells in a black, flat-bottomed 96-well plate and were then scanned on a microplate fluorometer (2300, EnSpireMultilabel Reader, PerkinElmer, Boston, MA, USA) using an excitation wavelength of 365 nm and an emission wavelength of 450 nm (Bell et al. 2013).
Statistical analysis
The effects of CO2, soil resource supply (four levels; i.e., low water-low P; high water-low P; low water-high P; high water-high P), time, and their interactions on CO2 exchange metrics were assessed in a repeated-measures framework. We carried out linear mixed effects models using the "nlme" package in R version 3.1.0 (Pinheiro et al. 2018) to evaluate the effects of CO2, resource availability and time (as fixed factors) on NEE,GPP, Reco and WUE, while accounting for multiple measurements within each replicate by nesting time within pots (Zuur et al. 2009). To assess the effects of eCO2 on soil moisture (i.e. soil water savings), we used the estimates of soil water content calculated at each watering event in a linear mixed effects model, including CO2, resource supply, time and their interactions as fixed factors, and time nested within pot as a random factor. The main effects of CO2, resource supply and their interactions on plant and rhizosphere-related metrics were assessed using two-way analysis of variance in the “stats” package in R version 3.1.0. All analyses were checked for residual normality and variance homoscedasticity, and log-transformations were applied when necessary to meet analysis assumptions. Post-hoc differences between CO2 levels at each resource addition treatment were examined for all models with Tukey’s Honest Significant Difference test for multiple comparisons using the “lsmeans” function within the “lsmeans” package in R version 3.1.0 (Russell 2016). For further analysis of changes in plant communities in response to experimental treatments see Ochoa-Hueso et al. (2021). Briefly, previous results from this experiment showed that P addition was the strongest driver of plant community composition at harvest time, although the effect of water was also significant. In contrast, community composition was not significantly affected by eCO2 (Table S1, Ochoa-Hueso et al. 2021).
Finally, we carried out structural equation modelling (SEM; Grace 2006) to evaluate the direct and indirect effects of eCO2, water and P on time-averaged, pot-level net ecosystem exchange (NEE), gross primary productivity (GPP) and respiration (Reco). Our a priori model (Fig. S1) also included those plant and soil properties that are known to control CO2 exchange rates in natural plant communities such as plant biomass (both aboveground and belowground), leaf tissue N and microbial activity in the rhizosphere (Phillips et al. 2011). We included rhizosphere enzyme activities as surrogates of microbial activity (Sinsabaugh et al. 2008). To obtain a quantitative index for the seven enzymes, we first normalized (log-transformed when needed) and standardized each of them (i.e., BG, CB, AG, XYL, NAG, LAP, PHOS) using the z-score transformation. Then, we parameterized our a priori model using our data set and tested its overall goodness-of-fit. To test the goodness-of-fit of the model, we used the chi-square test (the model has a good fit when 0.5 < P ≤ 1) and Bollen–Stine bootstrap (the model had a good fit when 0.1 < bootstrap P ≤ 1; Grace, 2006). To aid with the final interpretation of our SEMs, we also calculated the standardized total effects of the categorical predictors and plant and soil attributes on each component of ecosystem CO2 exchange (GPP, Reco). Total standardized effects of a given variable upon another were calculated by summing all direct and indirect pathways between these two variables (Grace 2006). All statistical analyses were performed using R 3.1.0 (R Development Core Team 2017).
Results
Plant production and community composition
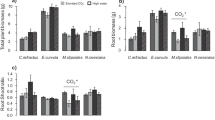
Overall, eCO2 did not increase total biomass, but rather it stimulated aboveground biomass by 10% (P < 0.001, Table 1) and decreased root biomass by 7% (P < 0.001, Table 1), consequently reducing the root-to-shoot ratio by 5% (P < 0.001, Table 1). However, these effects were highly dependent on soil resource availability. For example, compared to ambient, eCO2 increased total plant biomass under high P and low water conditions (+ 7%, P = 0.02, Table 2, Fig. 1), but not under high levels of both water and P (P = 0.14, Table 2, Fig. 1), despite an evident trend toward greater biomass. This was due to consistently lower biomass allocation belowground under eCO2 with large CO2-associated reductions in root mass at both low levels of water and P (i.e. wp treatment-16%; P = 0.004; Table 2, Fig. 1) and at high levels of these resources (i.e. WP treatment -12%; P = 0.01; Table 2, Fig. 1). Species level aboveground biomass data revealed treatment-dependent shifts in the C3:C4 ratio of plant communities. Phosphorus addition resulted in a trend towards increased C3:C4 ratios (p-value = 0.069, Table 2, Fig. S3), although water and CO2 treatments did not alter C3:C4 ratios. Post-hoc analyses showed that eCO2 increased C3:C4 ratio relative to ambient concentrations under the combination of low water treatment and high P.
Average of total, aboveground and belowground biomass for each of the CO2, water and P treatments. wp = low water and low P, Wp = high water and low P, wP = low water and high P, WP = high water and high P. Error bars represent ± 1 SE (n = 8). Asterisks indicate significant differences (P < 0.05) between CO2 levels from pairwise comparisons
Rhizosphere extracellular enzyme activity
There were no overall effects of eCO2 on rhizosphere enzyme activities when they were grouped according to their target element (i.e. C-enzymes, N-enzymes and phosphatase), nor were there interactions with soil resource supply (Table 3). This was confirmed by pairwise comparisons, but we observed a trend towards higher enzyme activities under eCO2 with high levels of both P and water (e.g., C-enzymes increased under eCO2 compared to ambient, at high soil resource availability P = 0.07, Table 4). The activity of N-associated enzymes was increased by eCO2 at high levels of both water and P (+ 36% P = 0.04, Table 4), but not under the other combinations of soil resource supply. Despite these modest effects of eCO2 on enzyme activities, there were no CO2 treatment effects on enzyme stoichiometry under any level of soil resources (Tables 3 and 4, Fig. 2).
Water use efficiency and CO2 fluxes
Over the course of the experiment, we found greater SWC under eCO2 in pots receiving high-P supply (i.e. wP and WP; P = 0.02 and P < 0.001, respectively; Fig. S2), indicating that eCO2 induced soil water savings when P availability was increased. However, we did not find effects of CO2 on instantaneous values of WUE (measured during CO2 flux measurement campaigns), despite evidence of increasing WUE under eCO2 over time (CO2 x Time, P = 0.007, Table 5). Pairwise comparisons also revealed that positive effects of eCO2 on WUE were stronger when both additional water and P were supplied (WP; -12%; P = 0.04), particularly towards the end of the experiment (Table 6, Fig. 3).We did not find an overall effect of eCO2 on NEE (P = 0.29,Table 5). In contrast, we found higher CO2 uptake (i.e. more negative NEE) under eCO2 in pots receiving additional P (wP -21%, P = 0.01 Table 6, Fig. 3), although not under high levels of water supply (WP, P = 0.10, Table 6, Fig. 3). Ecosystem respiration (Reco) was generally lower under eCO2, particularly during the last two measurement campaigns (August 11th and August 29th; Table 5, Fig. 3). There were no significant overall effects of CO2 treatment on GPP at any soil resource level (Table 5), although there was a trend towards more negative GPP (i.e., more C uptake) under eCO2 in pots to which only P was added (wP; Table 6, Fig. 3).
Direct vs indirect effects of eCO2 on key ecosystem functions (a Structural Equation Model)
In our SEM, eCO2, water supply and P fertilization each exerted control over different ecosystem functions. Elevated CO2 concentrations influenced NEE via a decrease in Reco (Fig. 4), high water supply directly enhanced rhizosphere enzyme activity, and P fertilization had positive effects on plant growth, both aboveground and belowground (Fig. 4). Despite P addition not having a direct effect on GPP or Reco, total standardized effects were significant for GPP, indicating greater CO2 uptake at high P availability. Water also exerted indirect control on Reco, likely mediated by its feedbacks on rhizosphere enzymatic activity. Surprisingly, rhizosphere enzyme activity was not significantly linked with metrics of CO2 exchange, or with CO2 treatment, either directly or indirectly. There were, however, indirect links between P fertilization and enzyme activity, likely through the positive effects of P addition on plant biomass.
a) Direct effects CO2, water and P on aboveground (Abv) and belowground (Blw) biomass, leaf N (%), rhizosphere enzymatic activity, gross primary productivity (GPP) and ecosystem-level respiration (Resp). Black arrows indicate positive effects and red dashed arrows indicate negative effects at P < 0.05. Grey arrows indicate positive (solid) and negative (dashed) effects at P < 0.1.Numbers adjacent to arrows indicate the effect size of the relationship. R2 denotes the proportion of variance explained. b) Standardized indirect and total (direct plus indirect) effects derived from the structural equation model, including the effects of CO2, water, P, aboveground and belowground biomass, leaf % N and rhizosphere enzymatic activity on gross primary productivity (GPP) and respiration (Resp)
Discussion
In this study, we grew an understorey plant community emerging naturally from its native seed-bank from a local, P-limited Eucalyptus woodland to explore the role of soil resource availability in moderating plant biomass responses to eCO2. We provide experimental evidence that positive effects of increased atmospheric [CO2] on plant production and ecosystem C dynamics are strongly constrained by low levels of P availability, and that modest increases in rainfall are unlikely to have a major impact on these responses. Our data also suggest that plant and microbial mechanisms that increase nutrient release from SOM may not be able to overcome this P limitation (Piñeiro et al. 2022). Although we recognise that there are limitations in extrapolating findings from a glasshouse experiment to field situations, this study nevertheless highlights the role of several important plant and microbial mechanisms influencing terrestrial feedbacks to the carbon cycle.
Our findings indicate that P supply is the main growth-limiting resource in our study system, as well as a major driver of community composition (Ochoa-Hueso et al., 2021), reflecting the importance of P limitation on primary productivity that has been recognised in several studies elsewhere (Peñuelas et al. 2013; Jiang et al. 2020; Piñeiro et al. 2022). The positive effect of P supply on plant biomass was greater under eCO2, with the eCO2 fertilization effect being negligible in the absence of P addition. The interactive effects of eCO2 and P on productivity might have been mediated by changes in C3:C4 ratio of plants, as P supply and eCO2 lead to a greater proportion of C3 relative to C4 species. This is consistent with the general hypothesis that eCO2 promotes C3 over C4 species, albeit in our experiment this selection for C3 species seems to be constrained by low P availability. Overall, the lack of eCO2 fertilization effect is in line with field-scale observations at EucFACE and appears to be independent of water availability, at least when severe water limitation (i.e. drought) is not involved (Duursma et al. 2016; Collins et al. 2018; Jiang et al. 2020). Despite the clear role of P availability in the composition and productivity responses to CO2, the absence of a CO2 fertilisation response for total plant biomass under high levels of both P and water leads us to reject our first hypothesis. This is largely because of the reduction in root biomass under eCO2 and high levels of soil resources, compared to ambient CO2, that counteracted positive aboveground responses (Dieleman et al. 2012; Piñeiro et al. 2020). Reduced investment in belowground biomass, or lower root: shoot ratios under eCO2 have been reported in other studies where irrigation removed any soil moisture limitation (Roumet et al. 1996; Volk et al. 2000). Our data therefore suggest that shifts towards greater investment in root biomass under eCO2 are more likely when soil moisture levels are low (Volk et al. 2000; Piñeiro et al. 2020). This is in line with conclusions from several meta-analyses that the effects of eCO2 on belowground biomass are modest, if any (Poorter and Nagel 2000; Poorter et al. 2012).
The lack of effect of our water treatment on overall plant biomass, and the absence of water x CO2 interactions, is in agreement with hypotheses H1a and H1b. Target water levels were chosen to explore the potential role of prolonged periods of high soil water content, likely resulting from larger rainfall events and greater variability in rainfall predicted for the end of this century at the study site (Webb & Hennessy, 2015). Our results highlight that such changes are unlikely to have a major impact on the CO2 fertilization effect. In drier (e.g. semi-arid) ecosystems, the positive feedbacks of eCO2 on plant production are attributed to enhanced WUE and concomitant soil water savings (Volk et al. 2000; Morgan et al. 2004), neither of which were generally affected by eCO2 in our experiment in the absence of P addition. These results are in line with those observed at the EucFACE field site, where greater WUE was not observed in the dominant understorey species (Pathare et al. 2017). The important role of soil moisture as a modifier of biomass responses to eCO2 has been identified in studies elsewhere (Wullschleger et al. 2002; Morgan et al. 2004), where relative biomass responses to eCO2 become greater during drier than average years. While our results highlight the key role of P supply at triggering plant physiological adaptations to eCO2 that might enhance biomass responses to increasing CO2 concentrations (e.g. improved WUE), we note that there is still the possibility of interactions between eCO2 and soil water that can be independent of P supply. This can be the case during growing seasons that experience long intervals between rainfall events and thus periods of low SWC where enhanced WUE is more likely to promote a stimulation of plant production under eCO2 (Silva and Lambers 2021).
Our second hypothesis—that eCO2 would increase rhizosphere enzyme activity under low P and water supply, as a mechanism for increasing nutrient availability—was not supported. Extracellular enzyme activities were similar to those reported in other studies (Phillips et al. 2011; Henry 2012), including under field conditions at the EucFACE experiment (Ochoa-Hueso et al. 2017). However, in contrast to findings from EucFACE, where eCO2 altered the activity of starch- (i.e. CB) and cellulose- (i.e. BG) degrading enzymes, we found negligible effects of eCO2 on rhizosphere enzyme activity. These differences may be due to the very low levels of soil water that occurred prior to the earlier field sampling at EucFACE (< 0.1 VWC; Ochoa-Hueso et al. 2017) that contrast strongly with those in our experiment. Indeed, water availability was the main factor driving rhizosphere microbial activity in our study, a finding that is in line with observations of positive relationships across rainfall gradients (Sinsabaugh et al. 2008) and water manipulation experiments elsewhere (Keeler et al. 2009; Henry 2012). Under eCO2, the stimulation in activity of extracellular enzymes related to the decomposition of N-rich substrates was only seen when levels of water and P were high. This response is likely due to increases in both the relative demand for N under high P supply and greater microbial metabolism with higher levels of soil moisture. Further, we found no effect of eCO2 on enzyme stoichiometry under any soil resource level, which suggests that greater exudation of C-rich compound by fine roots did not occur under eCO2 (Phillips et al. 2011). In line with this hypothesis, we also did not find greater GPP under eCO2, likely limiting the ability of plants to allocate more assimilated C into rhizosphere foraging via enhanced exudation rates. These results are in contrast with observations under field conditions in the EucFACE experiment (Ochoa-Hueso et al. 2017) and in a glasshouse study involving individual understory plants (Piñeiro et al. 2022), where eCO2 increased the demand for N and P relative to C in some of the species studied. These contrasting results might be due to the multispecies nature of our experimental set up. Piñeiro et al. (2022) found changes in enzyme stoichiometric ratios, but those were highly species-specific and dependent upon soil water availability. Because we collected rhizosphere from multiple species in each pot, we believe that the variability among individual species might have masked the overall effects of eCO2 on enzyme stoichiometry. These results highlight that responses of individual species growing in isolation might not be directly translated to mixed plant communities.
Experiments combining both eCO2 and N fertilization often report increased microbial demand for P (expressed as greater phosphatase activity or reductions in microbial C:P enzyme ratios; Chung et al. 2007), as well as a lack of CO2-associated stimulation of root production (Dieleman et al. 2010). In our experiment, we found enhanced activity of N-related enzymes under eCO2 when both additional water and P were supplied, suggesting increased microbial demand for N. This response can be due to changes in the relative allocation between shoots and roots driven by greater water supply, with the uptake of N (resulting from enhanced N-enzyme activity) being directed to aboveground functions at the expense of root growth. Accordingly, modelling approaches suggest that over-investment into fine root biomass under higher nutrient availability might not be an effective strategy where such investment would fail to support the additional C costs of maintaining more fine root biomass (Dybzinski et al. 2015). Our SEM implies that the stimulation in microbial activity associated with the water addition treatment reduced the need for plants to invest in fine root biomass. This explanation also fits with the observed negative relationship between root biomass and leaf N% in our SEM. Interestingly, P addition alone did not increase microbial N demand, and suggests that greater fine root biomass is needed for more extensive soil exploration when N availability is low (Taylor et al. 2014).
Our final prediction, that community-level net ecosystem CO2 uptake would increase under eCO2, was only supported where pots received additional P. The more negative values for NEE (i.e. greater net C fixation) under these conditions reflect a reduction in pot-level respiration rather than a stimulation of GPP (also supported by our SEM), which is a more commonly observed response to eCO2 (Bachman et al. 2010). In this regard, early studies into the role of P supply on photosynthetic responses to eCO2 highlight the critical role of P limitation underlying photosynthetic inhibition when plants are exposed to eCO2 (Sage et al. 1989; Conroy et al. 1990). Our SEM suggests that although P supply did not directly affect net CO2 exchange, the positive effects on CO2 uptake might be mediated by increases in plant biomass (note the positive total effects of P treatment on GPP in the SEM analysis). Our findings of a lack of eCO2 effect on GPP contrast with observations from the EucFACE field experiment (Pathare et al. 2017; Jiang et al. 2020), where stimulation of leaf-level photosynthesis has been reported for both trees and understory grasses under eCO2. Such differences may reflect the fact that we report community-level GPP responses, which include a variety of species, and leaves that are exposed to varying fractions of light due to shelf-shading (Sage et al. 1989; De Pury and Farquhar 1997). Differences in photosynthetic responses to eCO2 are well known among species (Leakey et al. 2009; Pathare et al. 2017), as well as among leaves of contrasting age and canopy position, and likely explain the lack of GPP response to eCO2 under low levels of P availability in our study (De Pury and Farquhar 1997; Herrick and Thomas 2003). Interestingly, eCO2 consistently decreased community-level respiration across all treatments. Given that eCO2 had only small effects on microbial activity (as expressed in terms of enzyme activities), the reduction in system-level respiration can be attributed to lower levels of soil respiration (also reflecting reduced belowground biomass; see Dieleman et al. 2010 for a review) and/or leaf dark respiration (Azcon-Bieto et al. 1994). Reports on leaf-level responses to chronic exposure to eCO2 suggest that mitochondrial respiration can be inhibited under eCO2, particularly when N dilution is induced by elevated [CO2] (Drake et al. 1999). Interestingly, leaf N% was negatively related to Reco in the SEM analysis which, at a first glance, seems to contradict the latter statement. We believe, however, that this relationship is a simple consequence of the lower biomass (both above and belowground) of plants with higher leaf N% (i.e. those pots not receiving P supply), with biomass driving the observed lower pot-level plant respiration under eCO2. In addition, in the absence of higher water supply, these communities showed the lowest levels of microbial activity, which may have contributed to reductions in soil respiration as well.
Conclusions
Overall, our results provide novel evidence of the role of P-limitation in ecosystem responses to eCO2 in a native understorey community from a Eucalyptus woodland. Phosphorus directly affected productivity responses to eCO2 as well as net CO2 uptake, but the addition of water above average levels did not stimulate nutrient uptake despite higher levels of rhizosphere enzyme activity in wetter soils. Elevated CO2 consistently reduced community-level respiration, but had no overall effect on GPP, suggesting that these two processes are controlled by different mechanisms (i.e. physiological inhibition and complex interactions with soil resource availability, respectively). While current Earth System Models predict positive feedback responses of terrestrial ecosystems on C storage under eCO2, here we emphasize the importance of accounting for the widespread phenomenon of P-limitation and the regulatory role of water availability in such responses.
References
Azcon-Bieto J, Gonzalez-Meler MA, Doherty W, Drake BG (1994) Acclimation of Respiratory O2 uptake in green tissues of field-grown native species after long-term exposure to elevated atmospheric CO2. Plant Physiol 106:1163–1168. https://doi.org/10.1104/pp.106.3.1163
Bachman S, Heisler-White JL, Pendall E et al (2010) Elevated carbon dioxide alters impacts of precipitation pulses on ecosystem photosynthesis and respiration in a semi-arid grassland. Oecologia 162:791–802. https://doi.org/10.1007/s00442-009-1511-x
Baldocchi D (2003) Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: past, present and future. Glob Chang Biol 9:479–492. https://doi.org/10.1046/j.1365-2486.2003.00629.x
Bell CW, Fricks BE, Rocca JD, Steinweg JM, McMahon SK, Wallenstein MD (2013) High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J Vis Exp 81:e50961
Collins L, Bradstock RA, Resco de Dios V et al (2018) Understorey productivity in temperate grassy woodland responds to soil water availability but not to elevated [CO2]. Glob Chang Biol 24:2366–2376. https://doi.org/10.1111/gcb.14038
Conroy JP, Milham PJ, Reed ML, Barlow EW (1990) Increases in phosphorus requirements for CO2-enriched pine species. Plant Physiol 92:977–982. https://doi.org/10.1104/pp.92.4.977
Crous KY, Ósvaldsson A, Ellsworth DS (2015) Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilisation stimulates stem growth. Plant Soil 391:293–305. https://doi.org/10.1007/s11104-015-2426-4
Crous KY, Zaragoza-Castells J, Löw M et al (2011) Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: impacts of elevated atmospheric CO2 and summer drought. Glob Chang Biol 17:1560–1576. https://doi.org/10.1111/j.1365-2486.2010.02325.x
Daws MI, Standish RJ, Koch JM, Morald TK (2013) Nitrogen and phosphorus fertilizer regime affect jarrah forest restoration after bauxite mining in Western Australia. Appl Veg Sci 16:610–618. https://doi.org/10.1111/avsc.12046
De Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20:537–557. https://doi.org/10.1111/j.1365-3040.1997.00094.x
Dieleman WIJ, Luyssaert S, Rey A et al (2010) Soil [N] modulates soil C cycling in CO2-fumigated tree stands: a meta-analysis. Plant Cell Environ 33:2001–2011. https://doi.org/10.1111/j.1365-3040.2010.02201.x
Dieleman WIJ, Vicca S, Dijkstra FA et al (2012) Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob Chang Biol 18:2681–2693. https://doi.org/10.1111/j.1365-2486.2012.02745.x
Dijkstra F, Carrillo Y, Pendall E, Morgan J (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:216
Drake BG, Azcon-Bieto J, Berry J et al (1999) Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ 22:649–657. https://doi.org/10.1046/j.1365-3040.1999.00438.x
Duursma RA, Gimeno TE, Boer MM et al (2016) Canopy leaf area of a mature evergreen Eucalyptus woodland does not respond to elevated atmospheric [CO2] but tracks water availability. Glob Chang Biol 22:1666–1676. https://doi.org/10.1111/gcb.13151
Dybzinski R, Farrior CE, Pacala SW (2015) Increased forest carbon storage with increased atmospheric CO2 despite nitrogen limitation: a game-theoretic allocation model for trees in competition for nitrogen and light. Glob Chang Biol 21:1182–1196. https://doi.org/10.1111/gcb.12783
Filion M, Dutilleul P, Potvin C (2000) Optimum experimental design for Free-Air Carbon dioxide Enrichment (FACE) studies. Glob Chang Biol 6:843–854. https://doi.org/10.1046/j.1365-2486.2000.00353.x
Finzi AC, Abramoff RZ, Spiller KS et al (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21:2082–2094. https://doi.org/10.1111/gcb.12816
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Hasegawa S, Piñeiro J, Ochoa-Hueso R et al (2018) Elevated CO2 concentrations reduce C4 cover and decrease diversity of understorey plant community in a Eucalyptus woodland. J Ecol 106:1483–1494. https://doi.org/10.1111/1365-2745.12943
Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59. https://doi.org/10.1016/j.soilbio.2011.12.026
Herrick JD, Thomas RB (2003) Leaf senescence and late-season net photosynthesis of sun and shade leaves of overstory sweetgum (Liquidambar styraciflua) grown in elevated and ambient carbon dioxide concentrations. Tree Physiol 23:109–118. https://doi.org/10.1093/treephys/23.2.109
Jiang M, Medlyn BE, Drake JE et al (2020) The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580. https://doi.org/10.1038/s41586-020-2128-9
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15. https://doi.org/10.1007/s10021-008-9199-z
Kolari P, Pumpanen J, Kulmala L et al (2006) Forest floor vegetation plays an important role in photosynthetic production of boreal forests. For Ecol Manage 221:241–248. https://doi.org/10.1016/j.foreco.2005.10.021
Körner C, Morgan J, Norby R (2007) CO2 Fertilization: When, where, how much? Terrestrial Ecosystems in a Changing World. Springer, Berlin, pp 9–21
Kuzyakov Y (2010) Priming effects: Interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Landuyt D, De Lombaerde E, Perring MP et al (2019) The functional role of temperate forest understorey vegetation in a changing world. Glob Chang Biol 25:3625–3641. https://doi.org/10.1111/gcb.14756
Laliberté E (2017) Below-ground frontiers in trait-based plant ecology. New Phytol 213:1597–1603. https://doi.org/10.1111/nph.14247
Leakey ADB, Ainsworth EA, Bernacchi CJ et al (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60:2859–2876. https://doi.org/10.1093/jxb/erp096
Leuzinger S, Hättenschwiler S (2013) Beyond global change: lessons from 25 years of CO2 research. Oecologia 171:639–651. https://doi.org/10.1007/s00442-012-2584-5
Morgan JA, Pataki DE, Körner C et al (2004) Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25. https://doi.org/10.1007/s00442-004-1550-2
Norby RJ, De Kauwe MG, Domingues TF et al (2016) Model-data synthesis for the next generation of forest free-air CO 2 enrichment (FACE) experiments. New Phytol 209:17–28. https://doi.org/10.1111/nph.13593
Norby RJ, Zak DR (2011) Ecological Lessons from Free-Air CO2 Enrichment (FACE) Experiments. Annu Rev Ecol Evol Syst 42:181–203. https://doi.org/10.1146/annurev-ecolsys-102209-144647
Ochoa-Hueso R, Carroll R, Piñeiro J, Power SA (2021) Understorey plant community assemblage of Australian Eucalyptus woodlands under elevated CO2 is modulated by water and phosphorus availability. J Plant Ecol 14:478–490. https://doi.org/10.1093/jpe/rtab005
Ochoa-Hueso R, Hughes J, Delgado-Baquerizo M et al (2017) Rhizosphere-driven increase in nitrogen and phosphorus availability under elevated atmospheric CO2 in a mature Eucalyptus woodland. Plant Soil 416. https://doi.org/10.1007/s11104-017-3212-2
Ochoa-Hueso R, Manrique E (2014) Impacts of altered precipitation, nitrogen deposition and plant competition on a Mediterranean seed bank. J Veg Sci 25:1289–1298. https://doi.org/10.1111/jvs.12183
Pathare VS, Crous KY, Cooke J et al (2017) Water availability affects seasonal CO2 -induced photosynthetic enhancement in herbaceous species in a periodically dry woodland. Glob Chang Biol 23:5164–5178. https://doi.org/10.1111/gcb.13778
Peñuelas J, Poulter B, Sardans J et al (2013) Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4:2934. https://doi.org/10.1038/ncomms3934
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194. https://doi.org/10.1111/j.1461-0248.2010.01570.x
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) _nlme: linear and nonlinear mixed effects models_. R package version 3.1–137. https://CRAN.R-project.org/package=nlme
Piñeiro J, Ochoa-Hueso R, Delgado-Baquerizo M et al (2017) Effects of elevated CO2 on fine root biomass are reduced by aridity but enhanced by soil nitrogen: A global assessment. Sci Rep 7. https://doi.org/10.1038/s41598-017-15728-4
Piñeiro J, Ochoa-Hueso R, Drake JE et al (2020) Water availability drives fine root dynamics in a Eucalyptus woodland under elevated atmospheric CO2 concentration. Funct Ecol 34:2389–2402. https://doi.org/10.1111/1365-2435.13660
Piñeiro J, Pathare V, Ochoa-Hueso R et al (2022) No CO2 fertilization effect on plant growth despite enhanced rhizosphere enzyme activity in a low phosphorus soil. Plant Soil 471:359–374. https://doi.org/10.1007/s11104-021-05175-6
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Funct Plant Biol 27:1191
Poorter H, Niklas KJ, Reich PB et al (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104-009-9895-2
Roumet C, Bel MP, Sonie L et al (1996) Growth response of grasses to elevated CO2: a physiological plurispecific analysis. New Phytol 133:595–603. https://doi.org/10.1111/j.1469-8137.1996.tb01928.x
Russell VL (2016) Least-Squares Means: The R Package lsmeans. J Stat Softw 69:1–33
Sage RF, Sharkey TD, Seemann JR (1989) Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiol 89:590–596. https://doi.org/10.1104/pp.89.2.590
Silva LCR, Lambers H (2021) Soil-plant-atmosphere interactions: structure, function, and predictive scaling for climate change mitigation. Plant Soil 461:5–27. https://doi.org/10.1007/s11104-020-04427-1
Sinsabaugh RL, Lauber CL, Weintraub MN et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Taylor BN, Strand AE, Cooper ER et al (2014) Root length, biomass, tissue chemistry and mycorrhizal colonization following 14 years of CO2 enrichment and 6 years of N fertilization in a warm temperate forest. Tree Physiol. https://doi.org/10.1093/treephys/tpu058
Volk M, Niklaus PA, Körner C (2000) Soil moisture effects determine CO2 responses of grassland species. Oecologia 125:380–388. https://doi.org/10.1007/s004420000454
Webb LB, Hennessy K (2015) Projections for selected Australian cities. CSIRO and Bureau of Meteorology, Australia
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2 - Implications for water-limited environments. Plant, Cell Environ. https://doi.org/10.1046/j.1365-3040.2002.00796.x
Wurth MKR, Winter K, Korner C (1998) In situ responses to elevated CO2 in tropical forest understory plants. Funct Ecol 12:886–895. https://doi.org/10.1046/j.1365-2435.1998.00278.x
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects modelling for nested data. Mixed effects models and extensions in ecology with R. Springer New York, New York, NY, pp 101–142
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piñeiro, J., Ochoa-Hueso, R., Serrano-Grijalva, L. et al. Phosphorus and water supply independently control productivity and soil enzyme activity responses to elevated CO2 in an understorey community from a Eucalyptus woodland. Plant Soil 483, 643–657 (2023). https://doi.org/10.1007/s11104-022-05763-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05763-0