Abstract
Background and aims
Common bean (Phaseolus vulgaris L.) nodulates with a wide range of rhizobia. Amongst these is Bradyrhizobium, which is inefficient but able to induce profuse nodulation on this crop. Based on this observation, we tested whether co-inoculating bradyrhizobia with a more standard common bean symbiont, Rhizobium tropici, could stimulate growth and nodulation of common bean, thus contributing to a more effective symbiosis.
Methods
Rhizobium tropici was co-inoculated with two Bradyrhizobium strains applied at three different doses (104, 106, and 108 CFU seed−1) under sterile conditions, and at a single dose (108 CFU seed−1) in non-sterile soil. Plant biomass, nodulation, and N accumulation in plant tissues were evaluated.
Results
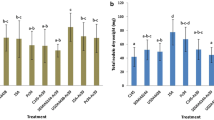
Co-inoculated plants produced more nodules, and accumulated more shoot dry biomass and nitrogen than plants inoculated with R. tropici alone under gnotobiotic conditions. Significant responses were observed at the highest inoculum dose and a significant correlation between dose and shoot dry weight was observed. Co-inoculation increased biomass and N accumulation in non-sterile soil, although with a smaller magnitude.
Conclusions
Altogether, our findings suggest that the co-inoculation with bradyrhizobia contributed to an improved symbiotic interaction between R. tropici and common beans.






Similar content being viewed by others
Abbreviations
- CFU:
-
Colony-forming unit
- PGPR:
-
Plant growth-promoting rhizobacteria
- EPS:
-
Exopolysaccharides
- LPS:
-
Lipopolysaccharides
- CPS:
-
Capsular polysaccharides
References
Ahmadzadeh M, Tehrani AS (2009) Evaluation of fluorescent pseudomonads for plant growth promotion, antifungal activity against Rhizoctonia solani on common bean, and biocontrol potential. Biol Control 48:101–107
Andrade D de S, Hamakawa PJ (1994) Estimativa do número de células viáveis de rizóbios no solo e em inoculantes por infecção em plantas. In: Hungria M, Araújo RS (eds) Manual de Métodos Empregados em Estudos de Microbiologia Agrícola. EMBRAPA-SPI, Brasília, pp 62–94
Andrade DS, Hungria M (2002) Maximizing the contribution of biological nitrogen fixation in tropical legume crops. In: Finan TM, O’Brian MR, Layzell DB, Vessey JK, Newton W (eds) Nitrogen fixation: global perspectives. CABI Publishing, Wallingford, pp 341–345
Baraúna AC, Rouws LF, Simoes-Araujo JL, dos Reis Junior FB, Iannetta PPM, Maluk M, Goi SR, Reis VM, James EK, Zilli JE (2016) Rhizobium altiplani sp. nov. isolated from effective nodules on Mimosa pudica growing in untypically alkaline soil in Central Brazil. Int J Syst Evol Microbiol 66:4118–4124
Boddey LH, Hungria M (1997) Phenotypic grouping of Brazilian Bradyrhizobium strains which nodulate soybean. Biol Fertil Soils 25:407–415
Brinkman EP, van der Putten WH, Bakker E-J, Verhoeven KJF (2010) Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J Ecol 98:1063–1073
Burdman S, Volpin H, Kigen J, Kapulnik Y, Okon Y (1996) Promotion of nod gene inducers and nodulation in common bean (Phaseolus vulgaris) roots inoculated with Azospirillum brasilense Cd. Appl Environ Microbiol 62(8):3030–3033
Camacho M, Santamaría C, Temprano F, Rodriguez-Navarro DN, Daza A (2001) Co-inoculation with Bacillus sp. cECT 450 improves nodulation in Phaseolus vulgaris L. Can J Microbiol 47:1058–1062
Canty A, Ripley B (2017) Boot: bootstrap R (S-Plus) functions. R package version 1.3–20
Cassán F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed gernimatino and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.) Eur J Soil Biol 45:28–35
Cumming G (2012) Understanding the new statistics: effect sizes, confidence intervals, and meta-analysis. Routledge, New York
D’Haeze W, Holsters M (2004) Surface polysaccharides enable bacteria to evade plant immunity. Trends Microbiol 12(12):555–561
Dall’Agnol RF, Plotegher F, Souza RC, Mendes IC, dos Reis Júnior FB, Béna G, Moulin L, Hungria M (2016) Paraburkholderia nodosa is the main N2-fixing species trapped by promiscuous common bean (Phaseolus vulgaris L.) in the Brazilian ‘Cerradão’. FEMS Microbiol Ecol 92(8):fiw108. https://doi.org/10.1093/femsec/fiw108
Dall’Agnol RF, Bournaud C, de Faria SM, Béna G, Moulin L, Hungria M (2017) Genetic diversity of symbiotic Paraburkholderia species isolated from nodules of Mimosa pudica (L.) and Phaseolus vulgaris (L.) grown in soils of the Brazilian Atlantic Forest (Mata Atlântica). FEMS Microbiol Ecol 93(4):fix027. https://doi.org/10.1093/femsec/fix027
Davison AC, Hinkley DV. (1997) Bootstrap Methods and Their Applications. Cambridge University Press, Cambridge
da Silva K, Florentino LA, da Silva KB, de Brandt E, Vandamme P, de Souza Moreira FM (2012) Cupriavidus necator isolates are able to fix nitrogen in symbiosis with different legume species. Syst Appl Microbiol 35(3):175–182
De Mendiburu F (2009) Una herramienta de analisis estadistico para la investigacion agricola. Tesis. Universidad Nacional de Ingenieria (UNI-PERU)
Delamuta JR, Ribeiro RA, Ormeño-Orillo E, Melo IS, Martínez-Romero E, Hungria M (2013) Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int J Syst Evol Microbiol 63(Pt 9):3342–3351. https://doi.org/10.1099/ijs.0.049130-0
Elliott GN, Chen W-M, Bontemps C, Chou J-H, Young JPW, Sprent JI, James EK (2007) Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Ann Bot 100:1403–1411
Ellis PD (2010) The essential guide to effect sizes: statistical power, meta-analysis, and the interpretation of research results. Cambridge University Press, Cambridge
Fox J, Weisberg S (2011) An {R} companion to applied regression, Second Edition. Sage, Thousand Oaks. URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion
Fraysse N, Couderc F, Poinsot V (2003) Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur J Biochem 270:1365–1380
Freire LR, Balieiro FC, Zonta E, dos Anjos, LC, Pereira MG, Lima E, Guerra JGM, Ferreira MBC, Leal MAA, De Campos DVB, Polidoro JC (2013) Manual de calagem e adubação do Estado do Rio de Janeiro. Brasília: Embrapa; Seropédica, RJ: Editora Universidade Rural 430 p
Gano-Cohen KA, Stokes PJ, Blanton MA, Wendlandt CE, Hollowell AC, Regus JU, Kim D, Patel S, Pahua VJ, Sachs JL (2016) Nonodulating Bradyrhizobium spp. modulate the benefits of legume-Rhizobium mutalism. Appl Environ Microbiol 17:5259–5268
Ghosh PK, Maiti TK (2016) Structure of extracellular polysaccharides (EPS) produced by rhizobia and their functions in legume-bacteria symbiosis: a review. Achiev Live Sci 10:136–143
Graham PH, Parker CA (1964) Diagnostic features in the characterization of the root-nodule bacteria of legumes. Plant Soil 20:383–396
Hungria M, Mendes IC (2015) Nitrogen fixation with soybean: the perfect symbiosis? In: de Bruijn FJ (ed) Biological Nitrogen Fixation. John Wiley & Sons, Inc, Hoboken. https://doi.org/10.1002/9781119053095.ch99
Hungria M, Joseph CM, Phillips DA (1991) Rhizobium nod gene inducers exuded naturally from roots of common bean (Phaseolus vulgaris L.) Plant Physiol 97:759–764
Hungria M, Andrade DS, Chueire LMO, Probanza A, Guttierrez-Mañero FJ, Megías M (2000) Isolation and characterization of new efficient and competitive bean (Phseolus vulgaris L.) rhizobia from Brazil. Soil Biol Biochem 32:1515–1528
Hungria M, Campo RJ, Mendes IC (2003) Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol Fertil Soils 39:88–93. https://doi.org/10.1007/s00374-003-0682-6
Hungria M, Nogueira MA, Araujo RS (2013) Co-inoculation of soybeans and common beans with rhizobia and azospirilla: strategies to improve sustainability. Biol Fertil Soils 49:791–801
Ishizawa S (1954) Studies on the root-nodule bacteria of leguminous plants. II. The relationship between nodule bacteria and leguminous plants, part 1. From the view of nodule production. 2 cross-inoculation test. J Sci Soil Manure 24:297–302
Kirby KN, Gerlanc D (2013) BootES: an R package for bootstrap confidence intervals on effect sizes. Behav Res Methods 45:905–927. https://doi.org/10.3758/s13428-013-0330-5
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863. https://doi.org/10.3389/fpsyg.2013.00863
Leigh JA, Signer ER, Walker GC (1985) Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A 82:6231–6235
López-López A, Rogel-Hernández MA, Barois I, Ortiz Ceballos AI, Martínez J, Ormeño-Orillo E, Martínez-Romero E (2012) Rhizobium grahamii sp. nov., from nodules of Dalea leporina, Leucaena leucocephala and Clitoria ternatea, and Rhizobium mesoamericanum sp. nov., from nodules of Phaseolus vulgaris, siratro, cowpea and Mimosa pudica. Int J Syst Evol Microbiol 62:2264–2271
Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, Ronson CW, James EK, Stougaard J (2010) The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat Commun 1:10. https://doi.org/10.1038/ncomms1009
Marks BB, Megías M, Nogueira MA, Hungria M (2013) Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp. and Azospirillum brasilense inoculants with soybean and maize. AMB Express 3:21. https://doi.org/10.1186/2191-0855-3-21
Martínez E, Pardo MA, Palacios R, Cevallos MA (1985) Reiteration of nitrogen fixation gene sequences and specificity of Rhizobium in nodulation and nitrogen fixation in Phaseolus vulgaris. J Gen Microbiol 131:1779–1786
Martínez-Romero (2003) Diversity of Rhizobium-Phaseolus vulgaris symbiosis: overview and perspectives. Plant Soil 252:11–23
Martínez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P, Pardo MA (1991) Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol 41(3):417–426
Masson-Boivin C, Giraud E, Perret X, Batut J (2009) Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends Microbiol 17(10):458–466
Michiels J, Dombrecht B, Vermeiren N, Xi C, Luyten E, Vanderleyden J (1998) Phaseolus vulgaris is a non-selective host for nodulation. Microb Ecol 26:193–205
Minamisawa K (1990) Division of rhizobitoxine-producing and hydrogen-uptake positive strains of Bradyrhizobium japonicum by nifDKE sequence divergence. Plant Cell Physiol 31:81–89
Mostasso L, Mostasso FL, Dias BG, Vargas MAT, Hungria M (2002) Selection of bean (Phaseolus vulgaris L.) rhizobial strains for the Brazilian Cerrados. Field Crop Res 73:121–132
Mrabet M, Mnasri B, Romdhane SB, Laguerre G, Aouani ME, Mhamdi R (2006) Agrobacterium strains isolated from root nodules of common bean specifically reduce nodulation by Rhizobium gallicum. FEMS Microbiol Ecol 56:304–309
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 82(4):591–605
Norris DO, T’Mannetje L (1964) The symbiotic specialization of African Trifolium spp. in relation to their taxonomy and their agronomic use. East Afr Agric For J 29:214–235
O’Hara GW, Hungria M, Woomer P, Howieson JG (2016) Counting rhizobia. In: Howieson JG, Dilworth MJ (ed) Working with rhizobia. Australian Centre for International Agricultural Research, Canberra, pp 109–124. Available at http://aciar.gov.au/publication/mn173. Accessed 24 Mar 2017
Oldroyd GED, Downie JA (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59(1):519–546
Ormeño-Orillo E, Rogel MA, Lloret L, López A, Martínez J, Vinuesa P, Martínez-Romero E (2009) Rhizobial diversity in different land use systems in the rain forest of Los Tuxtlas, Mexico. In: Barois I, Huising EJ, Okoth P, Trejo D, de Los Santos M (ed) Below-ground biodiversity in Sierra Santa Marta, Los Tuxtlas, Veracruz, México. Instituto de Ecología, A.C. Xalapa, Ver., México, p 65–84, 262 p
Perret X, Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev 64(1):180–201
Quelas JI, Mongiardini EJ, Casabuono A, López-García SL, Althabeboiti MJ, Covelli JM, Pérez-Giménez J, Couto A, Lodeiro AR (2010) Lack of galactose or galacturonic acid in Bradyrhizobium japonicum USDA 110 exopolysaccharide leads to different symbiotic responses in soybean. MPMI 23(12):1592–1604
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL https://www.R-project.org/
Ribeiro RA, Rogel MA, López-López A, Ormeño-Orillo E, Barcellos FG, Martínez FG, Martínez J, Thompson FL, Martínez-Romero E, Hungria M (2012) Reclassification of Rhizobium tropici type a strains as Rhizobium leucaenae sp. nov. Int J Syst Evol Microbiol 62:1179–1184
Rinella MJ, James JJ (2010) Invasive plant researchers should calculate effect sizes, not p-values. Invasive Plant Sci Manag 3:106–112
RStudio Team (2016) RStudio: integrated development for R. RStudio, Inc., Boston. URL. http://www.rstudio.com/
Somasegaran P, Hoben HJ (1985) Methods in legume-rhizobium technology. NifTal Hawaii, USA
Stanley JS, Brown GG, Verma DPS (1985) Slow-growing Rhizobium japonicum comprises two highly divergent symbiotic types. J Bacteriol 163:148–154
Talbi C, Delgado MJ, Girard L, Ramirez-Trujillo A, Caballero-Mellado J, Bedmar EJ (2010) Burkholderia phymatum strains capable of nodulating phaseolus vulgaris are present in moroccan soils. Appl Environ Microbiol 76(13):4587–4591
Tanaka K, Cho S-H, Lee H, Pham AQ, Batek JM, Cui S, Qiu J, Hkan SM, Joshi T, Zhang ZJ, Xu D, Stacey G (2015) Effect of lipo-chitooligosaccharide on early growth of C4 grass seedlings. J Exp Bot 66:5727–5738. https://doi.org/10.1093/jxb/erv260
Vacha-Haase T, Thompson B (2004) How to estimate and interpret various effect sizes. J Couns Psychol 51(4):473–481
Vlassak KM, Vanderleyden J, Graham PH (1997) Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci 16:163–229
Weaver RW, Danso SKA (1994) Dinitrogen fixation. SSSA book series, methods of soil analysis: part 2—microbiological and biochemical properties, 5.2, pp 1019–1045
Zgadzaj R, James EK, Kelly S, Kawaharada Y, de Jonge N, Jensen DB, Madsen LH, Radutoiu S (2015) A legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet 11(6):e1005280
Zuberer DA (1994) Recovery and enumeration of viable bacteria. In: Weaver RW, Angle JS, Bottomley PS (ed) Methods of soil analysis – part 2 – microbiological and biochemical properties, p 119–144
Acknowledgements
We would like to thank Dr. José Ivo Baldani and Dr. Robert Michael Boddey for reading the manuscript and giving suggestions to improve it. We also thank Dr. Andréia Loviane Silva for helping us with the acetylene reduction analysis; Wilson Cabral da Fonseca for helping us with microscopy sections; and Ernani Meirelles and his staff for giving support with the greenhouse experiments. We acknowledge the financial research support from Embrapa; the Coordination for the Improvement of Higher Education Personnel (Capes), which provided a Masters scholarship to Rafael de Almeida Leite; and the National Council for Scientific and Technological Development (CNPq), which provided a scholarship to Osnar Obede da Silva Aragão, and a research fellowship to Ederson da Conceição Jesus.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski .
Rights and permissions
About this article
Cite this article
Jesus, E.d., Leite, R.d., Bastos, R.d. et al. Co-inoculation of Bradyrhizobium stimulates the symbiosis efficiency of Rhizobium with common bean. Plant Soil 425, 201–215 (2018). https://doi.org/10.1007/s11104-017-3541-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3541-1