Abstract
Aims
We investigated whether drought-induced impairment of grassland species can be explained directly by plant water deficit or by water-driven limitation of nitrogen (N) and/or carbohydrate sources.
Methods
In a field experiment, a severe drought treatment was applied on monocultures of Lolium perenne L. (cv. Alligator) (Lp) and Trifolium repens L. (cv. Hebe) (Tr) by using rainout shelters excluding all precipitation, and effects were compared to a rainfed control. Three species-fertiliser treatments were set up, crossed with the drought treatment. The two species were fertilised equally with N (200 kg N ha−1 year−1), and an additional high N fertilisation treatment was established for L. perenne (LphighN, 500 kg N ha−1 year−1).
Results
Severe soil water deficit led to significantly lower leaf water potentials in all species-fertiliser treatments (P < 0.001) down to approximately −1.2 MPa and, on average, to a 79% reduction in living plant biomass above 7 cm harvest height (P < 0.001), indicating strong plant water deficits. Under the drought treatment, living plant biomass above 7 cm did not differ among species-fertiliser treatments. Plant-available soil N was 84% lower (P ≤ 0.01) and plant N concentrations were 24% less (P < 0.001) under the drought than under the rainfed control treatment, with Lp always being more N limited than LphighN and Tr. Nitrate concentrations in water-limited plants were generally very low (< 0.85 mg g−1 dry matter), whereas non-structural carbohydrates were distinctly greater under the drought treatment in Lp (+62%), LphighN (+46%), and Tr (+18%).
Conclusions
Restricted biomass production of these forage species under severe drought can primarily be explained by plant water deficits and secondarily by drought-induced limitation of N supply. However, growth seems not to be limited by carbohydrate source activity, as carbohydrates accumulated with water deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lolium perenne L. and Trifolium repens L. are the most widely grown forage species in intensively managed temperate grasslands in Europe, and due to their multiple benefits, grass-legume mixtures are considered a pillar of sustainable intensification of grassland systems (Lüscher et al. 2014; Suter et al. 2015). Both species are highly productive and provide high quality forage for ruminant production, but are known to be susceptible to severe drought events (Goh and Bruce 2005; Hofer et al. 2017). Drought events are projected to increase in frequency and severity in temperate regions due to climate change (Seneviratne et al. 2012; Trenberth et al. 2014), and experimental evidence suggests that highly productive grasslands could be more impaired by drought than low productivity grasslands (Pfisterer and Schmid 2002; Wang et al. 2007). An increased understanding of the drought-related mechanisms leading to yield losses of these economically important species could serve to improve their performance under conditions of soil water deficits through management and/or breeding practices.
Various essential growth factors can potentially become limited in response to drought, and the relationships among these are complex due to their interdependence (Farrior et al. 2013). Demonstrating a plant’s growth reaction to the limitation of several factors should give insight into the plant’s strategies for coping with stress. This can be achieved by simultaneously measuring growth-relevant source activities (e.g. uptake of water and nutrients, carbohydrate production) together with sink activities (biomass production, respiration) (Isopp et al. 2000; Roscher et al. 2011).
The effect of drought on soil water availability is usually most pronounced in the top soil layers (Hofer et al. 2016), and the shallow-rooted species L. perenne and T. repens might be particularly affected by persisting drought. Both species have about 90% of their root mass down to 20 cm deep (Hofer et al. 2017), and their water (and nitrogen) uptake seems to primarily occur in the top-most 10 cm of the soil (Hoekstra et al. 2014; Hoekstra et al. 2015; Prechsl et al. 2015). Where soil water uptake becomes restricted due to drought, leaf water potentials decrease (Skinner et al. 2004) and plants reduce their stomatal opening to diminish water losses and increase water use efficiency (Naudts et al. 2011). Aside from water, nitrogen (N) can become drought-limited (Colman and Lazenby 1975). Plant-available soil N is affected by drought through restricted water fluxes, reduced N mineralisation, and restricted transport of mineral N (Borken and Matzner 2009; Durand et al. 2010). Depending on the degree of drought severity, both water and N can simultaneously affect biomass production (Hooper and Johnson 1999); despite this being known, few studies have addressed drought-induced impacts on plant growth of other factors than water (but see Bollig and Feller 2014; Gonzalez-Dugo et al. 2005; Hofer et al. 2017). Recently, the relevance of N limitation under drought has been demonstrated at an induced five-week drought period, where N fertilisation partly mitigated negative effects of drought on biomass production in forage species (Hofer et al. 2017). Moreover, symbiotic N2 fixation in T. repens was maintained under drought, diminishing this species’ N limitation to some degree. However, Hofer et al. (2017) emphasised the need to investigate other plant metabolites such as nitrate and carbohydrates to fully assess the water, N and C balance under drought.
Regarding plant N metabolism, accumulation of nitrate in leaves may indicate an excess of plant-available soil N relative to N needed for growth (Isopp et al. 2000; Roscher et al. 2011), whereas a shortage in nitrate can be assumed under drought as a result of restricted N uptake and/or nitrate flow in the vascular system (Gonzalez-Dugo et al. 2010). With respect to carbohydrates, their production under ample water supply is mainly limited by light or soil nutrients (Roscher et al. 2011). Under drought, stomatal closure can lead to restricted carbon (C) dioxide exchange and C uptake (Bollig and Feller 2014; Naudts et al. 2011), which in turn can negatively affect carbohydrate source activity. Whether carbohydrates are more growth-limiting than water or soil N could be derived from the carbohydrates’ source-sink relation in the plant, which, in grassland species, is reflected in non-structural carbohydrates such as water-soluble carbohydrates (WSC) and starch (Isopp et al. 2000). Notably, patterns in the carbohydrates’ source-sink relation might differ between leaves (generally above 7 cm plant height in L. perenne and T. repens) and leaf sheaths and stems (L. perenne) or stolons and petioles (T. repens) (generally below 7 cm) (Fischer et al. 1997). Therefore, a full assessment of the plant’s source-sink balance needs the evaluation of both leaves (above 7 cm) and storage organs (below 7 cm).
Taken together, water, N, and carbohydrate sources, all of which are interrelated, could each potentially act as the primary limiting growth factor under drought. To assess the degree of limitation of each individual factor, we investigated monocultures of L. perenne and T. repens under induced drought during the second half of a ten-week simulated drought period under field conditions. Soil water and N availabilities were determined, and leaf water potential, stomatal conductance, and plant N were measured, while special attention was given to the source-sink balance of assimilated nitrate and non-structural carbohydrates. Nitrogen supply was modified by applying two levels of N fertilisation to L. perenne, while T. repens was considered to have additional N access through symbiotic N2 fixation (Hofer et al. 2017). We aimed to study whether water, N, or carbohydrate sources primarily are responsible for limited growth under severe drought and how these limitations are interconnected. The following hypotheses were tested:
-
I.
Severe drought leads primarily to water deficits in plants. Dominant water limitation is indicated by growth restrictions irrespective of N supply.
-
II.
Plant N limitation is enhanced under severe drought. Nitrogen limitation is stronger under low N supply (low N fertilised L. perenne) compared to high N supply (high N fertilised L. perenne, T. repens with N2 fixation).
-
III.
Drought impairs C-source more than C-sink, leading to a depletion of the level of non-structural carbohydrates. Depletion of non-structural carbohydrates is more evident under high N than under low N supply.
Materials and methods
Experimental setup and treatments
A field experiment was conducted in 2013 in the North-East of Switzerland near Zürich (see Hofer et al. 2017 for full details of site properties and establishment of the experiment). The soil at the site is classified as brown earth, with a top soil composition of 32% sand, 42% silt, 26% clay, and with a pH of 6.9. The shallow-rooted grass Lolium perenne L. (cultivar Alligator) and the shallow-rooted N2-fixing legume Trifolium repens L. (cultivar Hebe) were grown as monocultures in plots of 3 m × 5 m. A high N fertilisation treatment was set up for L. perenne only (see below for details on the treatments). Stands were established twice: under rainfed conditions (control) and under drought, in which a summer drought event was induced. Three replicates were set up for all treatments (L. perenne low and high N, T. repens; under rainfed control and drought; 18 plots in total), and plots were arranged in an incomplete block design.
A ten-week summer drought event was induced from June 5th to August 14th with precipitation being excluded completely for the drought treatment using rainout shelters. Shelters were 3 m × 5.5 m and covered with a transparent and ultraviolet light-transmissible plastic foil (see Hofer et al. 2016 for technical details). Sixty-four and 120 mm of precipitation were excluded during the first and the second half of the drought period, respectively, each half lasting for five weeks. Non-target effects of the shelters on air temperature (+0.9 °C, across the whole drought period) and relative humidity (−1.9%) were small, while the photosynthetic photon flux density above vegetation canopy was well beyond the threshold of light saturation of photosynthesis under control and drought conditions (see Table S1, Appendix 1, supplementary material, for further precipitation data and effects of the shelters on micro-meteorological variables). Hereafter, the term “drought” is used to refer to the rainout shelter treatment and its multiple effects on the biophysical environment. In contrast, “water deficit” and “water limitation” are used to specifically address the growth resource water as e.g. opposed to carbon or nitrogen.
All plots were managed following local practice of intensively managed grasslands and were harvested six times per year (plot harvester Hege 212, Wintersteiger, Austria), including harvests immediately before, in the middle, and at the end of the drought event (see Table S2 for harvest dates). A total amount of 200 kg N ha−1 year−1 was applied as ammonium-nitrate (Ammonsalpeter 27.5%, Lonza, Switzerland) for L. perenne (Lp) and T. repens (Tr), and 500 kg N ha−1 year−1 was used for the high N treatment for L. perenne (LphighN). Nitrogen was applied at the beginning of each of the six regrowth periods (see Table S2 for the detailed amount of N fertilisation per regrowth). Spontaneously establishing, unsown species were weeded regularly.
All measurements reported in this study took place during the second half of the drought period between July 10th and August 14th (hereafter referred to as the “measurement period”: days 36–70 after the onset of the drought treatment). Unless otherwise reported, four repeated measurements were taken over this period to investigate changes in variables with increasing duration of drought.
Measurements of resource availability: Soil water and nitrogen
Soil moisture content was measured in Lp and Tr plots twice per week at 5 cm and 40 cm soil depth under drought and control treatments using permanently installed sensors (n = 3 per species, depth, and drought treatment; EC-5 soil moisture sensor, Decagon, USA). Soil desorption curves (the relation between soil water content and soil matric potential) were determined for both soil depths from six representative plots using a standardised pressure plate method (Agroscope Reckenholz-Tänikon 2012). This provided a metric of the physical soil environment to quantify the plant-relevant water status (Vicca et al. 2012).
Plant-available soil N was measured in all plots with Plant Root Simulator (PRS)™ probes (Western Innovations, Canada) by imitating root nitrate and ammonium sorption (Qian and Schoenau 2005). Four anion and cation probes were vertically installed for seven days towards the end of the measurement period (days 62–69 after the onset of drought) at 5 cm and 15 cm soil depths (see Hofer et al. 2017 for further information on the use and analysis of the PRS™-probes).
Aboveground biomass
Aboveground biomass was measured in subplots of 40 cm × 40 cm, which were randomly located within the 5 m × 1.5 m central strip of each plot. Two types of samples were harvested with scissors: biomass above 7 cm plant height, mainly leaves, and biomass below 7 cm, mainly leaf sheaths and stems (L. perenne), and stolons and petioles (T. repens). Both samples were separated into living and dead material and freeze-dried (Gefriertrocknungsanlage Sublimator 3x4x5, Zirbus Technology, Germany) to determine dry matter.
Plant water and nitrogen
Leaf water potential was measured predawn under control and drought treatments (all plots) using a Scholander pressure chamber (Plant Moisture System SKPM 1400, Skye Instruments, UK) (n = 8 per plot, with measurements averaged for analysis). Stomatal conductance was measured three times in young, fully developed leaves using a porometer (SC-1 Leaf porometer, Decagon, USA) on sunny days after midday (n = 5 per plot, measurements averaged for analysis).
Plant N concentration was determined from dry matter samples separated as described (above and below 7 cm plant height, living and dead) by complete combustion using gas chromatography (Variomax CN Element Analyser, Elementar Analysensysteme, Germany) after grinding samples with a cutting mill (Muehle SM1, Retsch, Germany) to pass through a 0.75 mm sieve.
Carbohydrates and plant nitrate
Concentration of water-soluble carbohydrates (WSC) was determined in living biomass from above and below 7 cm plant height. In living biomass of T. repens, starch concentration was also measured to consider the conversion of excessive WSC into starch in legumes (Pelletier et al. 2010). For this purpose, plant samples were harvested in the morning around 8 am to catch remaining WSC not consumed overnight (Schnyder et al. 1988) and to reduce differences resulting from environment-dependent production during the day (Shewmaker et al. 2006) (see supplementary material, Appendix 1 for detailed information on harvesting and sample processing). Analysis of WSC and starch was performed using an anthrone method (Dreywood 1946) adapted following Fischer et al. (1997) and Trethewey and Rolston (2009). Water-soluble carbohydrates were extracted with water and ethanol, whereas starch was extracted using perchloric acid. After mixing and incubating the extracts with the anthrone reagent, photometric measurements (Spekol 1100, Zeiss, Germany) were taken. Based on glucose standards, WSC and starch concentrations were expressed as glucose equivalents (see supplementary material, Appendix 1 for detailed information on the chemical analysis). This allowed the summation of WSC and starch concentrations in Tr to represent total non-structural carbohydrates (TNC). In L. perenne, starch was not measured because it is a minor-to-negligible component of non-structural carbohydrates (Fischer et al. 1997).
Plant nitrate concentration was determined from samples taken subsequently to the collection of carbohydrate samples (see above) and following the same procedure as described (freeze-drying and grinding; supplementary material, Appendix 1). Extraction was conducted by combining 0.1 g of plant material, 4.8 ml deionised water and 0.2 ml trichloroacetic acid (12.5%) for 30 min, and after filtration, nitrate concentration was measured by ion chromatography (Dionex DX 500, Thermo Scientific, USA) using an anion-exchange column (Dionex IonPac™ AS4A-SC, Thermo Scientific, USA).
Data analysis
Living and dead biomass above and below 7 cm plant height, leaf water potential, stomatal conductance, concentrations of plant N, WSC, starch, and nitrate were analysed using linear mixed regression (Pinheiro and Bates 2000). Each model had the predictor variables species-fertiliser treatment (factor of three levels: Lp, LphighN, Tr), drought treatment (factor of two levels), measurement date (factor of four levels representing the repeated measurement dates), and their interactions. To account for repeated measurements over time, plot was specified as a random factor (random intercept). Data for plant-available soil N were only available from the end of the measurement period. Here, besides the species-fertiliser treatment and drought (as above), the model included the fixed variable soil depth (factor of two levels) and the random factor plot to account for correlation of soil depths within plots. Residuals of all models were checked to fulfil the assumptions of linear mixed regression. Data of plant-available soil N were natural log transformed prior to analysis to achieve homoscedasticity and normal distribution of residual variance. The model on plant nitrate included three variance terms for the species-fertiliser treatment to account for differing residual variance of the three strata (see Pinheiro and Bates 2000 p. 206 for details). Inference on specific contrasts related to our hypotheses was directly derived from the regression models (post-hoc t-tests, without applying multiple comparisons), and the summary tables of all regressions are provided in Appendix 1, supplementary material. All analyses were performed with the statistical software R (R Core Team 2017).
Results
Drought treatment induced low soil water
Soil moisture content (SMC) at 5 cm and 40 cm soil depths indicated generally ample water supply under rainfed control conditions, although it fluctuated with precipitation (Fig. 1). Under the drought treatment, however, SMC at 5 cm and 40 cm was clearly below a soil matric potential of −1.5 MPa for the entire measurement period (days 36–70 after installing the shelters). Soil moisture content was thus persistently below the approximate threshold of plant-accessible soil water, indicating severe water shortage in the main rooting zone of shallow-rooted species.
Soil moisture content (SMC) measured in L. perenne and T. repens plots at 5 cm (a) and 40 cm (b) soil depth under rainfed control and a drought treatment with complete rain exclusion. Displayed are means per control and drought treatments (n = 6) and mean SEs per measurement date. The dashed horizontal line is the SMC corresponding to a soil matric potential of −1.5 MPa, which is the approximate threshold for plant-accessible soil water
Low soil water induced strong plant water deficit
Predawn leaf water potentials under rainfed control conditions were between −0.2 and −0.7 MPa at all species-fertiliser treatments and measurement dates; by contrast, leaf water potentials under the drought treatment were between −1.0 and −1.5 MPa (Fig. 2), indicating strong plant water deficit (t = 8.76, P < 0.001, difference to the rainfed control, across measurement dates). By the end of the drought treatment, Lp and LphighN had a lower leaf water potential than Tr (t ≥ 2.59, P ≤ 0.024, day 70; Fig. 2); yet, N fertilisation did not influence leaf water potential of L. perenne (t = 0.44, P = 0.665, across measurement dates). In agreement with plant water limitation, leaf dry matter content in all species-fertiliser treatments was greater under the drought than under the control treatment (Fig. S1).
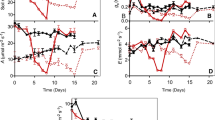
Stomatal conductance in Lp and LphighN was reduced by the drought treatment only towards the end of the measurement period (t ≥ 4.01, P ≤ 0.004, day 62; Fig. S2), whereas for Tr, the effect of the drought treatment on stomatal conductance was apparent at all measurement dates (t = 2.05, P = 0.110; Fig. S2, Fig. 3). Stomatal conductance was on average 5.3 times higher in Tr than in Lp and LphighN (t ≥ 8.37, P < 0.001, across measurement dates and drought treatments, Fig. 3), while the N fertilisation treatment in L. perenne did not consistently influence stomatal conductance (Table S4). Overall, there was a positive correlation between leaf water potential and stomatal conductance in T. repens (r = 0.49, P = 0.048, partial correlation coefficient given measurement date), but not in L. perenne (r = 0.11, P = 0.475).
Relationship between stomatal conductance and leaf water potential in L. perenne at two levels of N fertilisation (Lp, LphighN) and in T. repens (Tr) under rainfed control (Ctr) and a drought treatment with complete rain exclusion (Drt). Displayed are means (n = 3) ±1 SE; non-visible SEs are due to small size. Connected symbols reflect repeated measurements over time, and first measurements are indicated with #. See Fig. 2 and Fig. S2 for the corresponding graphs over the time axis
Plant water deficit strongly impaired biomass production independent of N supply
Under rainfed control conditions, living biomass above 7 cm plant height was 165 g m−2 for Tr at the end of the measurement period (Fig. 4a), but was only 83.9 and 84.2 g m−2 for Lp and LphighN, respectively, with no significant difference between the two N fertilisation levels (t = 0.02, P = 0.981, day 70). Under drought, all species-fertiliser treatments had very little living biomass above 7 cm (21 g m−2 on average), and there were no differences among the three species-fertiliser treatments (t ≤ 0.72, P ≥ 0.491, day 70). Living biomass below 7 cm of the Lp and LphighN treatments had, by the end of the measurement period, not been negatively affected by drought, whereas it was distinctly lower in Tr under drought compared to the rainfed control (t = 3.85, P = 0.003, day 70; Fig. 4b; Table S5: species-fertiliser × drought interaction). Dead biomass above and below 7 cm did not markedly differ between Lp and LphighN, and between control and drought treatments (Fig. 4c, d). However, near the end of the measurement period, dead biomass was greater in Tr under the drought compared to the control treatment above 7 cm (t = 2.76, P = 0.020, day 70; Fig. 4c) and below 7 cm (t = 2.02, P = 0.071, day 70; Fig. 4d; Table S5: species-fertiliser × drought interactions).
Dry matter of living (a, b) and dead (c, d) aboveground biomass above and below 7 cm of L. perenne at two levels of N fertilisation (Lp, LphighN) and of T. repens (Tr), under rainfed control (Ctr) and a drought treatment with complete rain exclusion (Drt). Displayed are means (n = 3; n = 2 for LphighN) and mean SEs per measurement date. Connected symbols reflect repeated measurements of biomass
Drought treatment caused low soil nitrogen
Plant-available soil N was, on average, 8.8 times lower for Lp compared to LphighN and Tr under rainfed control conditions (t ≥ 3.89, P ≤ 0.002, across both soil depths; Fig. 5). Under the drought treatment, plant-available soil N was distinctly lower than the control, irrespective of the soil depths (t = 3.08, P = 0.010, for Lp; t ≥ 6.95, P < 0.001, for LphighN and Tr; across both soil depths). Thus, plant-available soil N in Lp, LphighN, and Tr under drought was about two times lower than in Lp under rainfed conditions, and did not differ among the species-fertiliser treatments (t ≤ 1.43, P ≥ 0.180). No significant differences in plant-available soil N were found between the two soil depths (t = 0.21, P = 0.837, across species-fertiliser and drought treatments).
Plant-available soil N for L. perenne at two levels of N fertilisation (Lp, LphighN) and for T. repens (Tr) at 5 cm and 15 cm soil depth under rainfed control and a drought treatment with complete rain exclusion, measured with Plant Root Simulator (PRS)™ probes at the end of the measurement period (days 62 to 69 after onset of drought). Displayed are means (n = 3) ±1 SE. Non-visible SEs are due to small size, and inference is based on natural log transformed data, ***P ≤ 0.001, **P ≤ 0.01
Drought treatment reduced plant nitrogen
Nitrogen concentrations in living biomass above 7 cm under control conditions were generally higher in Tr and LphighN (43.1 g kg−1 DM on average) than in Lp (28.1 g kg−1 DM; t ≥ 7.80, P < 0.001, across measurement dates), and were comparably lower in all species-fertiliser treatments under drought (t = 7.85, P < 0.001, Fig. S3 and Fig. 6, Table S7). Consequently, Tr and LphighN also had higher N concentrations (32.2 g kg−1 DM on average) than Lp under the drought treatment (21.5 g kg−1 DM; t ≥ 7.80, P < 0.001, across measurement dates; Fig. S3). Under drought, all species-fertiliser treatments had N concentrations below the threshold for non-N-limited growth of 48 g N kg−1 DM at biomass below 100 g m−2 (Fig. 6, dashed line).
Relationship between N concentration and living biomass above 7 cm in L. perenne at two levels of N fertilisation (Lp, LphighN) and in T. repens (Tr) under rainfed control (Ctr) and a drought treatment with complete rain exclusion (Drt). Displayed are means (n = 3) ±1 SE; non-visible SEs are due to small size. Connected symbols reflect repeated measurements over time, and first measurements are indicated with #. Note that N concentration below 48 g N kg−1 dry matter (DM) indicates N limitation for biomass below 100 g m−2 in L. perenne and T. repens, and that above 100 g DM m−2 the critical threshold decreases with increasing biomass in a power function (threshold line indicated by – – –, following Duru et al. 1997; Gastal and Lemaire 2002). DM: dry matter. See Fig. 4 and Fig. S3 for the corresponding graphs over the time axis
No apparent plant carbohydrate limitation, but low nitrate concentrations in living biomass
Concentrations of water-soluble carbohydrates (WSC) in living biomass of Lp above 7 cm were significantly higher under the drought (135 mg g−1 DM on average) than under the control treatment (85 mg g−1 DM; t = 4.52, P < 0.001, across measurement dates). However, no significant difference in WSC between drought treatments was apparent for LphighN (t = 1.45, P = 0.173) and for total non-structural carbohydrates (TNC) in Tr (t = 0.86, P = 0.409) (Fig. 7a). Similarly, in living biomass below 7 cm, WSC in Lp was significantly higher under the drought (285 mg g−1 DM) than under the control treatment (174 mg g−1 DM; t = 6.39, P < 0.001). Here, however, the same pattern was observed in LphighN, with significantly higher WSC under drought (177 mg g−1 DM) than under control (109 mg g−1 DM; t = 4.33, P = 0.001) and for TNC in Tr (drought: 248 mg g−1 DM, control: 197 mg g−1 DM; t = 3.12, P = 0.009) (Fig. 7b). Regarding N fertilisation in L. perenne, levels of WSC in biomass below 7 cm were lower in LphighN compared to Lp under both control (t = 3.96, P = 0.002, across measurement dates) and drought treatments (t = 5.83, P < 0.001).
Concentration of water-soluble carbohydrates (WSC) in L. perenne at two levels of N fertilisation (Lp, LphighN) and concentration of total non-structural carbohydrates (TNC) in T. repens (Tr) for living biomass above (a) and below 7 cm (b) under rainfed control (Ctr) and a drought treatment with complete rain exclusion (Drt). Displayed are means (n = 3) and mean SEs per measurement date. Connected symbols reflect repeated measurements. DM: dry matter
Nitrate concentrations in living biomass above and below 7 cm were generally very low (≤ 0.85 mg g−1 DM) under rainfed control and drought treatments, except for the control of LphighN (up to 1.82 mg g−1 DM) (Fig. 8, Fig. S4). Nevertheless, in living biomass below 7 cm, Lp had significantly lower nitrate concentrations than LphighN and Tr under the control (t ≥ 6.35, P < 0.001, across measurement dates) and the drought treatment (t ≥ 3.12, P ≤ 0.009; Fig. S4). Overall, this resulted in a negative correlation with WSC concentrations across all three species-fertiliser treatments in living biomass above 7 cm (r = −0.33, P = 0.005, partial correlation coefficient given measurement date; Fig. 8a) and below 7 cm (r = −0.52, P < 0.001; Fig. 8b). Thus, additional N supply in LphighN and Tr resulted in higher nitrate but lower WSC concentrations while the drought treatment had generally the reversed effect, particularly in living biomass below 7 cm (compare Figs. 7b and 8b, Fig. S4b).
Relationship between concentrations of plant nitrate and water-soluble carbohydrates (WSC) in L. perenne at two levels of N fertilisation (Lp, LphighN) and concentration of total non-structural carbohydrates (TNC) in T. repens (Tr) for living biomass above (a) and below 7 cm (b) under rainfed control (Ctr) and a drought treatment with complete rain exclusion (Drt). Displayed are means (n = 3) ±1 SE; non-visible SEs are due to small size. Connected symbols reflect repeated measurements over time, and first measurements are indicated with #. DM: dry matter. See Fig. 7 and Fig. S4 for the corresponding graphs over the time axis
Discussion
Assessing resource supply and uptake under the severe drought treatment applied at field conditions and the resulting source-sink relations of two plant metabolites indicated for L. perenne and T. repens (i) a predominant plant water deficit, which severely restricted aboveground biomass production, (ii) a drought-induced reduction of plant N supply, resulting in N co-limitation, but (iii) no carbohydrate limitation of growth relative to water and nitrate.
Severe drought led to predominant plant water deficit
Very low or no soil water was plant-accessible in the top soil layer and at 40 cm depth during the entire measurement period, which was the second half of a ten-week drought period (Fig. 1). The top soil layer of 0–10 cm represents the main rooting zone for L. perenne and T. repens (Hofer et al. 2017) and their zone of major water uptake (Hoekstra et al. 2014). Consequently, water supply of plants under the shelters was strongly restricted during the entire measurement period, and the observed leaf water potentials of approximately −1.2 MPa suggest that leaf hydraulic conductivity strongly declined (Holloway-Phillips and Brodribb 2011). This lack of soil water was combined with a warm summer climate: during the measurement period, 12 days had temperature maxima of more than 30 °C, resulting in high water demand of the plants. Despite this, only T. repens reacted with a reduction in stomatal conductance under the drought treatment to diminish water losses, which resulted in a correlation to leaf water potential only within this species (Fig. 3). However, stomatal conductance under drought was still 4.6 times greater in T. repens than in L. perenne, which is in agreement with previous studies demonstrating generally higher stomatal conductance in T. repens compared to other non-legume species (Bollig and Feller 2014; Signarbieux and Feller 2012). Thus, in combination with our results of carbohydrate reserve accumulation under drought, this indicates that in particular T. repens could maintain a significant C uptake, even under strong water shortage.
Plant-available soil N was strongly reduced by the drought treatment, independent of species-fertiliser treatment and soil depth. Following Hofer et al. (2017), plant-available soil N as measured in this study reveals the balance between soil mineral N sources and mineral N uptake by the plants and microorganisms. Thus, the greater N availability measured in the highly fertilised L. perenne (LphighN) and in T. repens under rainfed control conditions (Fig. 5) suggests incomplete N uptake from the soil due to relatively high N availability from fertiliser, soil N mineralisation, and/or symbiotic N2 fixation. Under the drought treatment, however, the consistently low levels of plant-available soil N in all species-fertiliser treatments suggest severe inhibition of soil N mineralisation and/or of mineral N fluxes due to very low levels of soil water (Borken and Matzner 2009; Burke et al. 1997; Durand et al. 2010). Restrictions in the availability of mineral soil N must have been pervasive under drought as they could not be offset by additional N fertilisation of 60 kg ha−1 to L. perenne (Lp vs. LphighN), a clear contrast to rainfed control conditions (Fig. 5). Therefore, N uptake by plants must have been inhibited under drought, as indicated by N concentrations distinctly below the threshold for non-N-limited growth (approximately 50 g N kg−1 dry matter at low biomass of L. perenne and T. repens, Duru et al. 1997; Gastal and Lemaire 2002) in all three species-fertiliser treatments throughout the entire measurement period (Fig. 6, Fig. S3). Under rainfed control conditions, L. perenne fertilised at low N was already N limited (see Nyfeler et al. 2011, with extended discussion on N limitation), but limitation was further enhanced under the drought treatment. In contrast, the legume T. repens had generally the highest N concentrations with values above the N limitation threshold, most probably due to symbiotic N2 fixation. Under severe soil water limitation as experienced in this experiment, percent N derived from N2 fixation in T. repens was still around 55% (Hofer et al. 2017), indicating substantial N access through this pathway. While under rainfed conditions, N derived from N2 fixation is generally much higher, the value of 55% can be explained by severe limitation of other growth resources than soil N and concurrent down-regulation of symbiotic N2 fixation due to a reduced N demand of the plant (Divito and Sadras 2014; Hartwig 1998).
Together, this points to a predominant soil and plant water limitation under the five-week measurement period, which was at the end of a ten-week drought period. Recently, Hofer et al. (2017) found that N fertilisation of 30 kg ha−1 during an initial five-week drought period enhanced biomass production of L. perenne, indicating a degree of N limitation that could be offset by additional fertilisation. Thus, while N sources may primarily be growth-limiting at the onset of a drought period and only moderate water shortage, our data suggest that plant water deficit is predominant when soil water limitation becomes severe (Hofer et al. 2017; Hooper and Johnson 1999). It is possible that predominantly water-limited conditions could mitigate or even offset N limitation due to less N demand for growth. This, however, was not observed in our study: severe water limitation resulted in lower N concentrations in all species-fertiliser treatments compared to the rainfed control (Fig. 6). Our system under severe drought might therefore be specified as “predominantly water-limited, yet N co-limited”.
Strong plant water deficit led to cessation of plant biomass production
As a result of drought-induced restrictions in water and N supply, living plant biomass (above and below 7 cm) did not increase under the drought treatment during the entire measurement period (Fig. 4a, b); however, the dead fraction increased, particularly in T. repens. This indicates that similar amounts of biomass were produced than died under drought; yet, it also implies that plant metabolism and growth were maintained at least at low levels, because the increasing dead fraction must have been replaced by newly formed tissue. Following Volaire et al. (2014), plants are exposed to a trade-off between maintaining biomass resistance under moderate drought and surviving under severe drought. Plant responses leading to resistance under moderate drought occur through the maintenance of growth and require avoidance of leaf dehydration. Conversely, plant responses resulting in survival under severe drought are mainly associated with cessation of growth and dehydration tolerance in meristematic tissues. Yet, a strategy promoting growth under moderate drought might not necessarily be successful under severe drought, as represented in our experiment. In this light, the reaction of T. repens is particularly interesting. This species had larger stomatal conductance and produced more living biomass than L. perenne under rainfed control conditions (Fig. 4 b) and also exhibited superior growth under moderate drought (Hofer et al. 2016), most probably resulting from its benefit of symbiotic N2 fixation (Hofer et al. 2017). Thus, T. repens seems well adapted to moderate drought events (soil matric potentials below −1.5 MPa for less than 30 days, Hofer et al. 2017) where N limitation plays a major role. Under the drought treatment in the present study, inducing severe soil water limitation with soil matric potentials below −1.5 MPa for more than 40 days at both 10 cm and 40 cm soil depths, T. repens maintained the same growth strategy and demonstrated comparably higher stomatal conductance throughout the measurement period (Fig. 3), higher leaf water potentials than L. perenne specifically towards the end of the measurement period (Fig. 2), and largely maintained symbiotic N2 fixation for N supply (Hofer et al. 2017). However, this came at the price of high amounts of dead biomass above and below 7 cm (Fig. 4c, d), with the dead fraction being 97% and 124% of living biomass, respectively. This indicates severe tissue dehydration in T. repens (Lucero et al. 1999). Thus, while T. repens might have an advantage over L. perenne under moderate drought events and predominant N limitation, it seems severely and at least equally impaired like the grass species under increasing water shortage and severe drought, a reaction that has recently been shown in a multi-site study (Hofer et al. 2016). These results also indicate that the applied drought treatment resulted in an extreme stress for these fast growing, shallow-rooted forage species adapted to humid temperate climate.
Excessive carbohydrate source relative to limitation by water and nitrogen
Our measurements on plant growth and metabolic variables were generally split into the fractions above and below a plant height of 7 cm, which was done to distinguish leaves and storage organs, respectively. In both fractions, similar or higher WSC and TNC concentrations in water-limited compared to non-stressed plants were found, with the increase in carbohydrates being generally larger in biomass below 7 cm, i.e. in storage organs (Fig. 7). Such concentrations might even underestimate total carbohydrate storage because, under strong plant water deficit, an increasing proportion of assimilated carbohydrates are incorporated in roots relative to shoots (Burri et al. 2014). The lack of direct measurements of photosynthesis does not allow to conclude on drought-induced effects on absolute rates of C uptake. However, it can be concluded that carbohydrate source (photosynthesis) was less restricted than carbohydrate sinks for e.g. growth, metabolism, and symbiotic N2 fixation, resulting in excess C stored as reserves. Correspondingly, an early study found that photosynthesis only starts to decrease with leaf water potentials around −1.0 MPa, while leaf elongation was already strongly inhibited at these water potentials (Boyer 1970). Given the comparably low living biomass at all species-fertiliser treatments under drought, our results therefore support the hypothesis that leaf expansion in plants is inhibited by water limitation earlier and more severely than C metabolism itself (Muller et al. 2011). Further support for this hypothesis comes from additional measurements in our experiment: the leaf area index was considerably low under the drought treatment (< 0.3; Fig. S5), and concurrently, a large fraction of photosynthetic photon flux density was observed at ground level in all species-fertilisation treatments (> 60% of incident light at the end of the measurement period, Fig. S6). This indicates that intraspecific shading was negligible and that light (for photosynthesis) was sufficiently available, but that leaf formation must have been strongly restricted. Altogether, our results suggest that carbohydrates were not acting as a limiting growth factor from the drought treatment as applied here, but must be assumed sink-limited due to considerably stronger restrictions of water and N.
Finally, the comparison of nitrate and WSC concentrations is the most sensitive indicator of the relative limitation of plant N and carbohydrates needed for growth (Fischer et al. 1997). Under ample water supply, nitrate concentrations strongly depend on N fertilisation and growth, and nitrate can even accumulate, i.e. become sink-limited, in the event of high N sources (Isopp et al. 2000). Under the drought treatment of our study, one would expect nitrate concentrations either to remain low (compared to rainfed conditions) due to restrictions in the availability of mineral soil N or to accumulate and become sink-limited when growth is ceased (Fig. 4a, b). We generally found very low nitrate concentrations in all species-fertiliser treatments under drought, suggesting no sink-limitation. However, the additional N supply in highly fertilised L. perenne and T. repens (through symbiotic N2 fixation) resulted in higher nitrate, but lower WSC concentrations (Fig. 8), and this reaction was more pronounced in storage organs than in leaves. This clearly supports that plant metabolism was maintained under the drought treatment, although living biomass no longer increased. Moreover, the negative correlation between nitrate and WSC concentrations confirms that water limitation and concurrent N co-limitation were the primary restrictions in plant biomass production, rather than the provision of carbohydrates.
Conclusions
Severe soil water limitation led to strong water deficits in plants and co-limitation of N supply, which restricted aboveground plant biomass production. The source-sink balance of nitrate and carbohydrates in leaves and storage organs suggests that mineral N availability and biomass production become restricted earlier than carbohydrate source and that plant metabolism is maintained even under severe water limitation. Breeding for cultivars that can maintain water and N uptake under drought as long as possible should be a primary objective for future research.
Change history
05 December 2017
The article Severe water deficit restricts biomass production of Lolium perenne L. and Trifolium repens L. and causes foliar nitrogen but not carbohydrate limitation, written by Daniel Hofer, Matthias Suter, Nina Buchmann and Andreas Lüscher.
References
Agroscope Reckenholz-Tänikon ART (2012) Referenzmethoden der Forschungsanstalten, Band 2: Bodenuntersuchungen zur Standort-Charakterisierung. Agroscope Reckenholz-Tänikon ART, Zürich, Switzerland
Bollig C, Feller U (2014) Impacts of drought stress on water relations and carbon assimilation in grassland species at different altitudes. Agric Ecosyst Environ 188:212–220. https://doi.org/10.1016/j.agee.2014.02.034
Borken W, Matzner E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob Change Biol 15:808–824. https://doi.org/10.1111/j.1365-2486.2008.01681.x
Boyer JS (1970) Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol 46:233–235. https://doi.org/10.1104/pp.46.2.233
Burke IC, Lauenroth WK, Parton WJ (1997) Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology 78:1330–1340. https://doi.org/10.1890/0012-9658
Burri S, Sturm P, Prechsl UE, Knohl A, Buchmann N (2014) The impact of extreme summer drought on the short-term carbon coupling of photosynthesis to soil CO2 efflux in a temperate grassland. Biogeosciences 11:961–975. https://doi.org/10.5194/bg-11-961-2014
Colman RL, Lazenby A (1975) Effect of moisture on growth and nitrogen response by Lolium perenne. Plant Soil 42:1–13. https://doi.org/10.1007/bf02186970
Divito GA, Sadras VO (2014) How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crops Res 156:161–171. https://doi.org/10.1016/j.fcr.2013.11.004
Dreywood R (1946) Qualitative test for carbohydrate material. Ind Eng Chem Anal Ed 18:499. https://doi.org/10.1021/i560156a015
Durand J-L, Gonzalez-Dugo V, Gastal F (2010) How much do water deficits alter the nitrogen nutrition status of forage crops? Nutr Cycl Agroecosyst 88:231–243. https://doi.org/10.1007/s10705-009-9330-3
Duru M, Lemaire G, Cruz P (1997) The nitrogen requirement of major agricultural crops - Grasslands. In: Lemaire G (ed) Diagnosis of the nitrogen status in crops. Springer, Berlin, pp 59–72
Farrior CE, Tilman D, Dybzinski R, Reich PB, Levin SA, Pacala SW (2013) Resource limitation in a competitive context determines complex plant responses to experimental resource additions. Ecology 94:2505–2517. https://doi.org/10.1890/12-1548.1
Fischer BU, Frehner M, Hebeisen T, Zanetti S, Stadelmann F, Lüscher A, Hartwig UA, Hendrey GR, Blum H, Nösberger J (1997) Source-sink relations in Lolium perenne L. as reflected by carbohydrate concentrations in leaves and pseudo-stems during regrowth in a free air carbon dioxide enrichment (FACE) experiment. Plant Cell Environ 20:945–952. https://doi.org/10.1046/j.1365-3040.1997.d01-131.x
Gastal F, Lemaire G (2002) N uptake and distribution in crops: an agronomical and ecophysiological perspective. J Exp Bot 53:789–799. https://doi.org/10.1093/jexbot/53.370.789
Goh KM, Bruce GE (2005) Comparison of biomass production and biological nitrogen fixation of multi-species pastures (mixed herb leys) with perennial ryegrass-white clover pasture with and without irrigation in Canterbury, New Zealand. Agri Ecosyst Environ 110:230–240. https://doi.org/10.1016/j.agee.2005.04.005
Gonzalez-Dugo V, Durand J-L, Gastal F, Picon-Cochard C (2005) Short-term response of the nitrogen nutrition status of tall fescue and Italian ryegrass swards under water deficit. Austral J Agric Res 56:1269–1276. https://doi.org/10.1071/ar05064
Gonzalez-Dugo V, Durand J-L, Gastal F (2010) Water deficit and nitrogen nutrition of crops. A review. Agron Sustain Develop 30:529–544. https://doi.org/10.1051/agro/2009059
Hartwig UA (1998) The regulation of symbiotic N2 fixation: A conceptual model of N feedback from the ecosystem to the gene expression level. Perspect Plant Ecol Evol Syst 1:92–120. https://doi.org/10.1078/1433-8319-00054
Hoekstra NJ, Finn JA, Hofer D, Lüscher A (2014) The effect of drought and interspecific interactions on depth of water uptake in deep- and shallow-rooting grassland species as determined by δ18O natural abundance. Biogeosciences 11:4493–4506. https://doi.org/10.5194/bg-11-4493-2014
Hoekstra NJ, Suter M, Finn JA, Husse S, Lüscher A (2015) Do belowground vertical niche differences between deep- and shallow-rooted species enhance resource uptake and drought resistance in grassland mixtures? Plant Soil 394:21–34. https://doi.org/10.1007/s11104-014-2352-x
Hofer D, Suter M, Haughey E, Finn JA, Hoekstra NJ, Buchmann N, Lüscher A (2016) Yield of temperate forage grassland species is either largely resistant or resilient to experimental summer drought. J Appl Ecol 53:1023–1034. https://doi.org/10.1111/1365-2664.12694
Hofer D, Suter M, Buchmann N, Lüscher A (2017) Nitrogen status of functionally different forage species explains resistance to severe drought and post-drought overcompensation. Agric Ecosyst Environ 236:312–322. https://doi.org/10.1016/j.agee.2016.11.022
Holloway-Phillips M-M, Brodribb TJ (2011) Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant Cell Environ 34:302–313. https://doi.org/10.1111/j.1365-3040.2010.02244.x
Hooper DU, Johnson L (1999) Nitrogen limitation in dryland ecosystems: Responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–293. https://doi.org/10.1023/a:1006145306009
Isopp H, Frehner M, Almeida JPF, Blum H, Daepp M, Hartwig UA, Lüscher A, Suter S, Nösberger J (2000) Nitrogen plays a major role in leaves when source-sink relations change: C and N metabolism in Lolium perenne growing under free air CO2 enrichment. Austral J Plant Physiol 27:851–858. https://doi.org/10.1071/PP99151
Lucero DW, Grieu P, Guckert A (1999) Effects of water deficit and plant interaction on morphological growth parameters and yield of white clover (Trifolium repens L.) and ryegrass (Lolium perenne L.) mixtures. Europ J Agron 11:167–177. https://doi.org/10.1016/s1161-0301(99)00028-3
Lüscher A, Mueller-Harvey I, Soussana JF, Rees RM, Peyraud JL (2014) Potential of legume-based grassland-livestock systems in Europe: a review. Grass Forage Sci 69:206–228. https://doi.org/10.1111/gfs.12124
Muller B, Pantin F, Genard M, Turc O, Freixes S, Piques M, Gibon Y (2011) Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J Exp Bot 62:1715–1729. https://doi.org/10.1093/jxb/erq438
Naudts K, van den Berge J, Janssens IA, Nijs I, Ceulemans R (2011) Does an extreme drought event alter the response of grassland communities to a changing climate? Environ Exp Bot 70:151–157. https://doi.org/10.1016/j.envexpbot.2010.08.013
Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Lüscher A (2011) Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric Ecosyst Environ 140:155–163. https://doi.org/10.1016/j.agee.2010.11.022
Pelletier S, Tremblay GF, Bélanger G, Bertrand A, Castonguay Y, Pageau D, Drapeau R (2010) Forage nonstructural carbohydrates and nutritive value as affected by time of cutting and species. Agron J 102:1388–1398. https://doi.org/10.2134/agronj2010.0158
Pfisterer AB, Schmid B (2002) Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416:84–86. https://doi.org/10.1038/416084a
Pinheiro JC, Bates DM (2000) Mixed-effects Models in S and S-Plus. Springer, New York, US
Prechsl UE, Burri S, Gilgen AK, Kahmen A, Buchmann N (2015) No shift to a deeper water uptake depth in response to summer drought of two lowland and sub-alpine C3-grasslands in Switzerland. Oecologia 177:97–111. https://doi.org/10.1007/s00442-014-3092-6
Qian PY, Schoenau JJ (2005) Use of ion-exchange membrane to assess nitrogen-supply power of soils. J Plant Nutr 28:2193–2200. https://doi.org/10.1080/01904160500324717
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Roscher C, Kutsch WL, Schulze ED (2011) Light and nitrogen competition limit Lolium perenne in experimental grasslands of increasing plant diversity. Plant Biol 13:134–144. https://doi.org/10.1111/j.1438-8677.2010.00338.x
Schnyder H, Nelson CJ, Spollen WG (1988) Diurnal growth of tall fescue leaf blades: II. Dry matter partitioning and carbohydrate metabolism in the elongation zone and adjacent expanded tissue. Plant Physiol 86:1077–1083. https://doi.org/10.1104/pp.86.4.1077
Seneviratne SI et al. (2012) Changes in climate extremes and their impacts on the natural physical environment. In: Field CB et al. (eds) Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge and New York, pp 109–230
Shewmaker GE, Mayland HF, Roberts CA, Harrison PA, Chatterton NJ, Sleper DA (2006) Daily carbohydrate accumulation in eight tall fescue cultivars. Grass Forage Sci 61:413–421. https://doi.org/10.1111/j.1365-2494.2006.00550.x
Signarbieux C, Feller U (2012) Effects of an extended drought period on physiological properties of grassland species in the field. J Plant Res 125:251–261. https://doi.org/10.1007/s10265-011-0427-9
Skinner RH, Gustine DL, Sanderson MA (2004) Growth, water relations, and nutritive value of pasture species mixtures under moisture stress. Crop Sci 44:1361–1369. https://doi.org/10.2135/cropsci2004.1361
Suter M, Connolly J, Finn JA, Loges R, Kirwan L, Sebastià M-T, Lüscher A (2015) Nitrogen yield advantage from grass-legume mixtures is robust over a wide range of legume proportions and environmental conditions. Glob Change Biol 21:2424–2438. https://doi.org/10.1111/gcb.12880
Trenberth KE, Dai A, van der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J (2014) Global warming and changes in drought. Nat Clim Chang 4:17–22. https://doi.org/10.1038/nclimate2067
Trethewey JAK, Rolston MP (2009) Carbohydrate dynamics during reproductive growth and seed yield limits in perennial ryegrass. Field Crops Res 112:182–188. https://doi.org/10.1016/j.fcr.2009.03.001
Vicca S et al (2012) Urgent need for a common metric to make precipitation manipulation experiments comparable. New Phytol 195:518–522. https://doi.org/10.1111/j.1469-8137.2012.04224.x
Volaire F, Barkaoui K, Norton M (2014) Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Europ J Agron 52:81–89. https://doi.org/10.1016/j.eja.2013.10.002
Wang Y, Yu S, Wang J (2007) Biomass-dependent susceptibility to drought in experimental grassland communities. Ecol Lett 10:401–410. https://doi.org/10.1111/j.1461-0248.2007.01031.x
Acknowledgements
The authors thank C. Stutz, R. Gago, M. Leuzinger, D. Bäder, L. Vögeli, and L. Weber for assistance with field work and sample processing, and T. Zehnder and S. van Geijtenbeek for the analysis of carbohydrates. We are grateful to A. Oberson Dräyer and two anonymous reviewers for helpful comments on an earlier version of this paper, and to K. Seipel for linguistic editing. Meteorological data were provided by the Federal Office for Meteorology (MeteoSwiss) and Plant Root Simulator (PRS)™ probes by Western AG Innovations. We acknowledge financial support by the AnimalChange project from the European Union’s Seventh Framework Programme (FP7/2007-2013; grant agreement no. 266018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory
The original version of this article was revised due to a retrospective Open Access order.
A correction to this article is available online at https://doi.org/10.1007/s11104-017-3508-2.
Electronic supplementary material
ESM 1
(PDF 692 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hofer, D., Suter, M., Buchmann, N. et al. Severe water deficit restricts biomass production of Lolium perenne L. and Trifolium repens L. and causes foliar nitrogen but not carbohydrate limitation. Plant Soil 421, 367–380 (2017). https://doi.org/10.1007/s11104-017-3439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3439-y