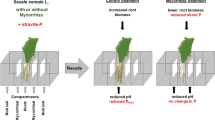
Abstract
Background and aims
Arbuscular mycorrhizal fungi (AMF) are plant root symbionts highly specialized upon phosphorus (P) supply to their hosts. We investigated plants’ ability to regulate carbon (C) flow to AMF depending on the soil P supply.
Methods
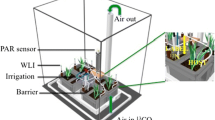
Leek (Allium porrum), medic (Medicago truncatula), and ryegrass (Lolium perenne) were subjected to AMF inoculation and/or P fertilization in a glasshouse experiment. The C flows were traced using 13C pulse labelling.
Results
Mycorrhizal P uptake responses were lowered by P fertilization in all tested plant species. Independently from the C flow to the roots, the C flow to AMF-signature fatty acid 16:1ω5 were reduced by P fertilization in leek and ryegrass (but not in medic). Calculated mycorrhizal C costs ranged between 0.9% and 10.5% of the plant C budget.
Conclusions
Suppression of the C flow from the plants to AMF resulted from both reduced abundance of AMF in the roots and lowered relative C income per unit of AMF biomass in P-fertilized pots. Although inconsistencies amongst different plant species demand caution in making generalizations, these results suggest an active role of host plants in regulating the C flow to AMF.




Similar content being viewed by others
References
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J 6:291–297
Bécard G, Piché Y (1989) Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl Environ Microbiol 55:2320–2325
Black KG, Mitchell DT, Osborne BA (2000) Effect of mycorrhizal-enhanced leaf phosphate status on carbon partitioning, translocation and photosynthesis in cucumber. Plant Cell Environ 23:797–809. doi:10.1046/j.1365-3040.2000.00598.x
Blanke V, Renker C, Wagner M, Füllner K, Held M, Kuhn AJ, Buscot F (2005) Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol 166:981–992. doi:10.1111/j.1469-8137.2005.01374.x
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants - an economic analogy. Annu Rev Ecol Syst 16:363–392. doi:10.1146/annurev.es.16.110185.002051
Bravo A, Brands M, Wewer V, Dormann P, Harrison MJ (2017) Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol 214:1631–1645
Calderón FJ, Schultz DJ, Paul EA (2012) Carbon allocation, belowground transfers, and lipid turnover in a plant-microbial association. Soil Sci Soc Am J 76:1614–1623. doi:10.2136/sssaj2011.0440
Casieri L et al (2013) Biotrophic transportome in mutualistic plant-fungal interactions. Mycorrhiza 23:597–625. doi:10.1007/s00572-013-0496-9
Douds DD, Johnson CR, Koch KE (1988) Carbon cost of the fungal symbiont relative to net leaf P accumulation in a split-root VA mycorrhizal symbiosis. Plant Physiol 86:491–496. doi:10.1104/pp.86.2.491
Drigo B et al (2010) Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. Proc Natl Acad Sci U S A 107:10938–10942. doi:10.1073/pnas.0912421107
Facelli E, Facelli JM, Smith SE, McLaughlin MJ (1999) Interactive effects of arbuscular mycorrhizal symbiosis, intraspecific competition and resource availability on Trifolium subterraneum cv. Mt. barker. New Phytol 141:535–547. doi:10.1046/j.1469-8137.1999.00367.x
Facelli E, Duan T, Smith SE, Christophersen HM, Facelli JM, Smith FA (2014) Opening the black box: outcomes of interactions between arbuscular mycorrhizal (AM) and non-host genotypes of Medicago depend on fungal identity, interplay between P uptake pathways and external P supply. Plant Cell Environ 37:1382–1392. doi:10.1111/pce.12237
Franken P (2010) Molecular-physiological aspects of the AM symbiosis post penetration. In: Kapulnik Y (ed) Koltai H. Arbuscular Mycorrhizas, Physiology and Function, pp 93–116. doi:10.1007/978-90-481-9489-6_5
Frostegård Å, Tunlid A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14:151–163. doi:10.1016/0167-7012(91)90018-l
Garcia K, Doidy J, Zimmermann SD, Wipf D, Courty PE (2016) Take a trip through the plant and fungal transportome of mycorrhiza. Trends Plant Sci 21:937–950. doi:10.1016/j.tplants.2016.07.010
Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE (2009) Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol 181:938–949. doi:10.1111/j.1469-8137.2008.02720.x
Graham JH, Hodge NC, Morton JB (1995) Fatty acid methyl ester profiles for characterization of glomalean fungi and their endomycorrhizae. Appl Environ Microbiol 61:58–64
Grimoldi AA, Kavanová M, Lattanzi FA, Schaufele R, Schnyder H (2006) Arbuscular mycorrhizal colonization on carbon economy in perennial ryegrass: quantification by 13CO2/12CO2 steady-state labelling and gas exchange. New Phytol 172:544–553. doi:10.1111/j.1469-8137.2006.01853.x
Grman E (2012) Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93:711–718
Harris D, Pacovsky RS, Paul EA (1985) Carbon economy of soybean-Rhizobium-Glomus associations. New Phytol 101:427–440. doi:10.1111/j.1469-8137.1985.tb02849.x
Jakobsen I, Rosendahl L (1990) Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol 115:77–83. doi:10.1111/j.1469-8137.1990.tb00924.x
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789. doi:10.1111/j.1469-8137.2007.02294.x
Ji BM, Bever JD (2016) Plant preferential allocation and fungal reward decline with soil phosphorus: implications for mycorrhizal mutualism. Ecosphere 7 doi:10.1002/ecs2.1256
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. doi:10.1111/j.1469-8137.2009.03110.x
Johnson NC, Graham JH (2013) The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 363:411–419. doi:10.1007/s11104-012-1406-1
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586. doi:10.1046/j.1469-8137.1997.00729.x
Johnson D, Leake JR, Read DJ (2002) Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: short-term respiratory losses and accumulation of 14C. Soil Biol Biochem 34:1521–1524. doi:10.1016/s0038-0717(02)00126-8
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205:1473–1484. doi:10.1111/nph.13172
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem 41:1233–1244. doi:10.1016/j.soilbio.2009.03.005
Kiers ET et al (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal Symbiosis. Science 333:880–882. doi:10.1126/science.1208473
Koch KE, Johnson CR (1984) Photosynthate partitioning in split-root Citrus seedlings with mycorrhizal and nonmycorrhizal root systems. Plant Physiol 75:26–30. doi:10.1104/pp. 75.1.26
Koide R, Elliott G (1989) Cost, benefit and efficiency of the vesicular-arbuscular mycorrhizal symbiosis. Funct Ecol 3:252–255
Kucey RMN, Paul EA (1982) Carbon flow, photosynthesis, and N2 fixation in mycorrhizal and nodulated faba beans (Vicia faba L.) Soil Biol Biochem 14:407–412. doi:10.1016/0038-0717(82)90013-x
Lemoine R et al (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4. doi:10.3389/fpls.2013.00272
Lendenmann M, Thonar C, Barnard RL, Salmon Y, Werner RA, Frossard E, Jansa J (2011) Symbiont identity matters: carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 21:689–702. doi:10.1007/s00572-011-0371-5
Li HY, Smith SE, Holloway RE, Zhu YG, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol 172:536–543. doi:10.1111/j.1469-8137.2006.01846.x
Liu H, Trieu AT, Blaylock LA, Harrison MJ (1998) Cloning and characterization of two phosphate transporters from Medicago truncatula roots: regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi. Mol Plant-Microbe Interact 11:14–22. doi:10.1094/mpmi.1998.11.1.14
Manck-Götzenberger J, Requena N (2016) Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front Plant Sci 7. doi:10.3389/fpls.2016.00487
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Miltner A, Kopinke FD, Kindler R, Selesi D, Hartmann A, Kästner M (2005) Non-phototrophic CO2 fixation by soil microorganisms. Plant Soil 269:193–203
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181:950–959. doi:10.1111/j.1469-8137.2008.02721.x
Neumann E, George E (2010) Nutrient uptake: the arbuscular mycorrhiza fungal symbiosis as a plant nutrient acquisition strategy. Arbuscular Mycorrhizas: Physiol Funct. doi:10.1007/978-90-481-9489-6_7
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895. doi:10.2136/sssaj1991.036159950055000300046x
Olsson PA, Johnson NC (2005) Tracking carbon from the atmosphere to the rhizosphere. Ecol Lett 8:1264–1270. doi:10.1111/j.1461-0248.2005.00831.x
Olsson PA, van Aarle IM, Gavito ME, Bengtson P, Bengtsson G (2005) 13C incorporation into signature fatty acids as an assay for carbon allocation in arbuscular mycorrhiza. Appl Environ Microbiol 71:2592–2599. doi:10.1128/aem.71.5.2592-2599.2005
Olsson PA, Rahm J, Aliasgharzad N (2010) Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol Ecol 72:123–131. doi:10.1111/j.1574-6941.2009.00833.x
Pearson JN, Jakobsen I (1993) The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants, measured by dual labeling with 32P and 33P. New Phytol 124:489–494. doi:10.1111/j.1469-8137.1993.tb03840.x
Poulsen KH, Nagy R, Gao LL, Smith SE, Bucher M, Smith FA, Jakobsen I (2005) Physiological and molecular evidence for pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytol 168:445–453. doi:10.1111/j.1469-8137.2005.01523.x
Püschel D, Janoušková M, Hujslová M, Slavíková R, Gryndlerová H, Jansa J (2016) Plant-fungus competition for nitrogen erases mycorrhizal growth benefits of Andropogon gerardii under limited nitrogen supply. Ecol Evol 6:4332–4346. doi:10.1002/ece3.2207
Řezáčová V, Gryndler M, Bukovská P, Šmilauer P, Jansa J (2016) Molecular community analysis of arbuscular mycorrhizal fungi - contributions of PCR primer and host plant selectivity to the detected community profiles. Pedobiologia 59:179–187. doi:10.1016/j.pedobi.2016.04.002
Řezáčová V, Konvalinková T, Jansa J (2017a) Carbon fluxes in mycorrhizal plants. In: Varma a, Prasad R, Tuteja N (eds) mycorrhiza - eco-physiology, secondary metabolites, nanomaterials. 4 edn. Springer International Publishing, p 335. doi:10.1007/978-3-319-57849-1_1
Řezáčová V, Slavíková R, Konvalinková T, Hujslová M, Gryndlerová H, Gryndler M, Püschel D, Jansa J (2017b) Imbalanced carbon-for-phosphorus exchange between European arbuscular mycorrhizal fungi and non-native Panicum grasses – a case of dysfunctional symbiosis. Pedobiologia 62:48–55
Schroeder MS, Janos DP (2004) Phosphorus and intraspecific density alter plant responses to arbuscular mycorrhizas. Plant Soil 264:335–348. doi:10.1023/b:plso.0000047765.28663.49
Schroeder MS, Janos DP (2005) Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 15:203–216. doi:10.1007/s00572-004-0324-3
Slavíková R et al (2017) Monitoring CO2 emissions to gain a dynamic view of carbon allocation to arbuscular mycorrhizal fungi. Mycorrhiza 27:35–51. doi:10.1007/s00572-016-0731-2
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edition. Mycorrhizal Symbiosis, 3rd edition
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524. doi:10.1111/j.1469-8137.2004.01039.x
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182:347–358. doi:10.1111/j.1469-8137.2008.02753.x
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057. doi:10.1104/pp.111.174581
Snellgrove RC, Splittstoesser WE, Stribley DP, Tinker PB (1982) The distribution of carbon and the demand of the fungal symbiont in leek plants with vesicular arbuscular mycorrhizas. New Phytol 92:75–87. doi:10.1111/j.1469-8137.1982.tb03364.x
Sochorová L, Jansa J, Verbruggen E, Hejcman M, Schellberg J, Kiers ET, Johnson NC (2016) Long-term agricultural management maximizing hay production can significantly reduce belowground C storage. Agric Ecosyst Environ 220:104–114. doi:10.1016/j.agee.2015.12.026
Valentine AJ, Osborne BA, Mitchell DT (2001) Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization, growth and photosynthesis of cucumber. Sci Hortic 88:177–189. doi:10.1016/s0304-4238(00)00205-3
Valentine AJ, Mortimer PE, Kleinert A, Kang Y, Benedito VA (2013) Carbon metabolism and costs of arbuscular mycorrhizal associations to host roots. In: Aroca R (ed) Symbiotic endophytes, vol 37. Soil biology, pp 233–252. doi:10.1007/978-3-642-39317-4_12
Verbruggen E, Jansa J, Hammer EC, Rillig MC (2016) Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil? J Ecol 104:261–269
Watts-Williams SJ, Jakobsen I, Cavagnaro TR, Grønlund M (2015) Local and distal effects of arbuscular mycorrhizal colonization on direct pathway pi uptake and root growth in Medicago truncatula. J Exp Bot 66:4061–4073. doi:10.1093/jxb/erv202
Welc M, Buenemann EK, Fliessbach A, Frossard E, Jansa J (2012) Soil bacterial and fungal communities along a soil chronosequence assessed by fatty acid profiling. Soil Biol Biochem 49:184–192. doi:10.1016/j.soilbio.2012.01.032
Werner GDA, Kiers ET (2015) Partner selection in the mycorrhizal mutualism. New Phytol 205:1437–1442. doi:10.1111/nph.13113
van de Wiel CCM, van der Linden CG, Scholten OE (2016) Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207:1–22
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738. doi:10.2307/2446507
Wyatt GAK, Kiers ET, Gardner A, West SA (2014) A biological market analysis of the plant-mycorrhizal symbiosis. Evolution 68:2603–2618. doi:10.1111/evo.12466
Acknowledgements
This work was supported by projects LK11224 and LO1417 granted by the Ministry of Education, Youth and Sports of the Czech Republic, by project 14-19191S granted by the Czech Science Foundation, and by the long-term development program RVO61388971. Constructive comments by three anonymous reviewers and the editor are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Tatsuhiro Ezawa.
Electronic supplementary material
ESM 1
(PDF 138 mb)
Rights and permissions
About this article
Cite this article
Konvalinková, T., Püschel, D., Řezáčová, V. et al. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 419, 319–333 (2017). https://doi.org/10.1007/s11104-017-3350-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3350-6