Abstract
Aims
The paper presents results from plot experiments aimed at the development of an ecological strategy for soil contaminated with mercury. Meadow grass (Poa pratensis) was tested on mercury contaminated soil in a former chlor-alkali plant (CAP) in southern Poland for its phytoremediation potential.
Methods
The stabilisation potential of the plants was investigated on plots without additives and after the addition of granular sulphur. Biomass production, uptake and distribution of mercury by plants, as well as leachates and rhizosphere microorganisms were investigated, along with the growth and vitality of plants during one growing season.
Results
The analysed plants grew easily on mercury contaminated soil, accumulating lower amounts of mercury, especially in the roots, from soil with additive of granular sulphur (0.5 % w/w) and sustained a rich microbial population in the rhizosphere. After amendment application the reduction of Hg evaporation was observed.
Conclusions
The obtained results demonstrate the potential of using Poa pratensis and sulphur for remediation of mercury contaminated soil and reduction of the Hg evaporation from soil. In the presented study, methods of Hg reduction on “hot spots” were proposed, with a special focus on environmental protection. This approach provides a simple remediation tool for large areas heavily contaminated with mercury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury and its compounds are considered to be potentially hazardous to all biological organisms (Asztalos et al. 2012; Boening 2000; Chen and Yang 2012). Mercury cannot be destroyed biologically but only transformed into volatile metallic mercury, HgO (Hobman and Brown 1997) or biomethylated by a number of bacterial species to gaseous methyl mercury (De et al. 2008) or dimethyl mercury (Rodriguez et al. 2005). In soil the element is mostly associated with humic acids forming strong complexes with sulphur-containing functional groups (Hooda 2010; Zuo et al. 2013). Mercury occurs naturally at trace levels in the earth’s crust, however, potentially toxic concentrations of mercury are continuously released into the environment from anthropogenic sources. For several decades chemical plant facilities in Poland (e.g. chlor-alkali production processes) as well as in other countries (Zagury et al. 2006; Dufault et al. 2009; Esbrí et al. 2014) have been using mercury and its compounds in manufacturing processes, which resulted in high concentrations of mercury in nearby soils. This poses potential health and environmental risk over a much wider area (Cachada et al. 2009; Pereira et al. 2009). The most popular remediation technologies for mercury contaminated soils are: liquid extraction, thermal treatment, electrolytic methods, mercury flotation or immobilisation and solidification/stabilisation (S/S). Some of the S/S technologies are based on Hg stabilisation and solidification with sulphur polymer cement, thiol-functionalised zeolite compounds and alkali sulphide (Fuhrmann et al. 2002; Piao and Bishop 2006; Bower et al. 2008; Zhang et al. 2009). These technologies are expensive, complex processes that are destructive for soil organisms and of limited application in soil treatment (Wang et al. 2012; Randall and Chattopadhyay 2013).
The less disruptive and more affordable alternatives based on phytoremediation approach use the physiological and biochemical possibilities of plants and the appropriate modification of soil compartment properties. Such an approach, based on the reduction of Hg bioavailable fractions, was investigated in the contaminated areas of the chlor-alkali plant in southern Poland. Poa pratensis was tested for its potential for aided phytostabilisation. Granular sulphur in concentration of 0.5 % w/w was used as a soil additive. The beneficial effect of sulphur on the retention of Hg in soil was first suggested by Lagerwerff (1967) and continued by Maclean (1974). According to Outridge et al. (2001) the direct interaction between Hg and S leads to the formation of metacinnabar or cinnabar. The environmental impact of sulphur addition to the Hg contaminated soil was tested via analysis of soil leachates for Hg, SO4 +2 and total organic carbon (TOC) concentrations as well as soil microorganisms.
Materials and methods
Experimental site
The experimental site was located inside the former chemical facility (southern Poland) that has been operating for over 50 years. For several decades the facility had been using mercury and its compounds in its manufacturing processes. Three sources of mercury emission to the atmosphere from the area were identified: chlorine production using electrolysis, production of acetaldehyde from acetylene and coal combustion in the facility’s power and heating plant. The sources of soil contamination include technical processes in chlor-alkali, acetaldehyde or vinyl chloride industries, where activated carbon with HgCl2 is used as a catalyst. The data from the long-term environmental monitoring programmes show that the contaminated area is limited only to the areas between the facilities.
Site characterisation
The area located between the former chlor-alkali plant facilities was chosen for the plot experiment. For soil characterisation representative soil samples (30 kg; 0–25 cm depth; zigzag pattern) from the selected area were collected and prepared for soil analysis and a treatability study.
Treatability study
Prior to establishing the scheme of plot experiments the treatability study on representative soil samples from the selected area was performed. Soil was collected at the level of 0–25 cm and sieved through a 4 mm grid for the pot experiment. In a lab-scale experiment pure and granular sulphur and zeolite (73.4 % SiO2 and 12.7 % of Al2O3) were incorporated into the Hg- contaminated soil in doses of 0.5 %, 1 % and 5 % w/w and the samples were tested for their stabilisation properties. As the most effective additive the 0.5 % w/w granular sulphur was chosen for the experiment. The selection was based on the data presented in Tables 1 and 2 relating to soil pH values and the effective reduction of water-soluble and exchangeable mercury compounds.
Experimental design
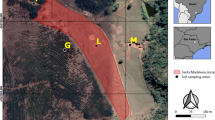
The site was prepared for the experiment and a natural plant cover with roots was extracted. Soil from the area designed for plot experiment was dug up (up to 25 cm depth), mixed and returned to the plot. Then fifteen subplots (9 m2 each) were established and prepared for planting. About 120 L of soil were transported to the lab for a Hg evapotranspiration experiment. Five samples were taken diagonally across each plot (surface layer 0–20 cm), mixed and analysed for soil characteristics. Plots were randomly selected before planting. Granular sulphur was incorporated to the soil depth of 15 cm at a concentration of 0.5 % w/w. Poa pratensis, which is the dominant species in the existing plant cover in CAPs, was used for the plot experiment. After two weeks (the experimentally verified time for geochemical soil stabilisation after an amendment application) Poa pratensis was planted from commercially available seeds (10 kg ha−1). The experimental plot design with randomly distributed variants (3 replications), was as follows (Fig. 1):
-
reference plots – no granular sulphur, no plants (C),
-
plots with granular sulphur (S),
-
plots with granular sulphur and Poa pratensis – (S + M ),
-
plots with Poa pratensis (M)
Physical and chemical properties of soil
Soil was sieved through a 1 mm grid for evaluation of soil properties and then ground in a mortar to pass through a 0.25 mm grid for mercury analyses. The following parameters were determined in the soil: the grain size distribution (the Casagrande’s aerometric method modified by Prószyński), pH in 1 M KCl (ratio 1:5, potentiometric method, according to ISO 10390:1997), soil electrical conductivity (EC) (ratio 1:5, conductivity measurement, according to ISO 11265:1997), total nitrogen (Ntot) percentage (Kjeldahl method, combustion and extraction in Buchi apparatus), content of organic phosphorus (P2O5) and potassium (K2O) (Egner-Riehm method) and organic carbon (Corg) (Tiurin’s method). For the determination of the cation exchange capacity (CEC) and the content of exchangeable cations (Ca2+, K+, Mg2+, Na+) in the soil, hexamminecobalt trichloride (Sigma-Aldrich) was used and the extractants were analysed by ICP-OES according to ISO 23470:2007.
Soil samples for mercury content were collected after geochemical stabilisation (two weeks after the amendment application – the start of the experiment) and at the end of the experiment (after 14 weeks). The soil samples (1 g) were transferred to Teflon vessels for mineralisation in a microwave oven (MDS 2000 (CEM)) using concentrated nitric acid and hydrochloric acid (1:1) (Hg ≤ 0.000001 %, pro analysis, Merck, Germany). Then the samples were heated in a microwave oven for 50 min at the temperature of 160 °C and pressure of 20 bar. The concentration of mercury was determined by the “cold vapour” atomic adsorption spectrometry with (SnCl2) as a reducing agent using AAS Spectra 300 P, Varian, VGA 76.
The procedure for sequential extraction of mercury was performed based on the methodology given by Di Giulio and Ryan (1987); Wallschläger et al. (1998) and Schwesig et al. (1999). The five-step sequential extraction scheme included the following fractions: water-soluble (F1), exchangeable (F2), fulvic and humic acids (F3), organic/sulphide (F4) and residual (F5). About 5 g of soil sample was extracted of eluent (ratio sample/eluent =1:10) respectively: deionised distilled water (F1), 1 M CH3COONH4 (F2), 1 M KOH (subsequent acidification to pH 1 ÷ 2 with HNO3-F3), 0.1 M HNO3 with H2O2 (F4) and digestion (85 °C) in aqua regia (F5). Each sample was shaken with extraction solvent for 1 h, centrifuged (3000 rpm for 10 min) and filtered through a 0.45 μm glass fibre filter to exclude particles and collect the supernatant. Mercury content in all fractions was determined by CVAAS (Varian Spectra AA 300P).
Sulphide concentration in the soil was calculated as a difference between mercury organic/sulphide fractions and Hg fractions bound to refractory organic matter (HNO3 extraction).
Plant analysis
Plants were harvested and washed with distilled water. Aerial parts were excised from the roots and dried separately at 40 °C. The plant samples (1 g) were transferred to Teflon vessels for mineralisation in a microwave oven (Microwave system MDS 2000 (CEM)) using 10 mL concentrated nitric acid and hydrochloric acid (1:1) (Hg ≤ 0.000001 %, pro analysis, Merck, Germany). The samples were heated in a microwave oven for 50 min at the temperature of 160 °C and pressure of 20 bar. Concentration of mercury was determined by the “cold vapour” atomic absorption spectrometry with (SnCl2) as a reducing agent using AAS Spectra 300 P, Varian, VGA 76.
Soil leachates
In order to determine the activity in the soil solution, Teflon vacuum cup lysimeters were installed on each plot at a depth of 25 cm. After rainfall events (six sampling events during the vegetation period) soil solution samples from lysimeters were collected and analysed for Hg2+ − determined by cold vapour AAS method using a flame atomic absorption spectrophotometer (Varian Spectra AA300 Mod. 5) and SO4 2− determined according to ISO 11304–1 method and TOC determined using PN-EN 1484 method.
Microbial investigation
For root-free soil analysis 10 g of soil was transferred to 250 mL Erlenmeyer flasks containing 90 mL 0.85 % NaCl. Flasks were shaken on a rotary shaker for 10 min at 120 rpm. The resultant soil suspension was used to determinate the root-free soil microbial population. Three individual samples for each single plot were collected at the end of the experiment and analysed separately (Barillot et al. 2012). Soil samples were examined to determine: the total number of bacteria, number of gram-negative bacteria, Pseudomonas, nitrate decomposing bacteria, sulphur amino-acid decomposing bacteria and the number of fungi. All tests on solid media (10 % TSA), selective medium for Pseudomonas (Grant and Holt 1977) and Streptomyces (Bacto Malt-Extract Agar) were determined by the surface plating technique using 0.1 mL of appropriately diluted sample. After serial dilution in 0.85 % NaCl, dilutions were plated onto 10 % tryptic soy agar (TSA) on the selective medium for Pseudomonas and on Czapek-Dox medium with rose bengal for soil fungi (Alef 1995).
The number of nitrate and sulphur amino-acid decomposing bacteria was estimated by the MPN (most probable number) method. The total number of colony forming units of soil bacteria (CFU g−1) was evaluated on 10 % tryptic soy agar – TSA (Difco). The total number of fungal colony forming units was determined based on the Czapek-Dox medium with rose bengal (Alef 1995). Colonies were counted after incubation at 28 °C for 3 days for growth of bacteria and at 22 °C for 7 days for growth of fungi. To determine the growth of bacteria the colonies were counted after incubation at 28 °C for 3 days and at 22 °C for 7 days for growth of fungi.
Ammonifying and sulphur amino-acid decomposing bacteria were determined on the level of 10 % peptone medium. The tested medium was inoculated with a 1 mL aliquot from each dilution of 10−1 through 10−7 of each soil samples (three test tubes). Inoculated test tubes were incubated in the dark at 28 ± 2 °C for 7 days. The presence of ammonia (Merckoquant tests) (Sas-Nowosielska et al. 2008) and hydrogen sulphide were recorded in each tube. The results were correlated with probability tables. The MPN of the bacterial population was expressed as counts per gram of dry soil (dw).
The data were presented as average values of among bacterial counts and the one-way analysis of variance (ANOVA, Statistica 10) was used for statistical analysis. The LSD (Least Significant Difference) test was used for pairwise comparisons of means at the 0.05 level.
Hg evaporation/volatilisation from the soil
Hg contaminated soil from the investigated area (Table 1) was prepared according to the experimental design presented in Fig. 1 and placed in pots (50 x 35 x 12 cm) in three replicates. Six pots were planted with Poa pratensis (10 kg ha−1). Hg evaporation was investigated on control pots and pots with sulphur. The effect of plant cover was measured in pots planted with Poa pratensis with and without additive (0.5 % of granular of sulphur).
Evaporation of Hg from soil was measured using a dynamic flux chamber method (DFC) according to Ferrara and Mazzolai (1998). The method allows for measuring the gaseous mercury concentration released from the soil surface to the atmosphere, inside a specially constructed glass chamber. The chamber (42 cm x 26 cm x 22 cm) was placed on each single pot on the soil surface (1 cm depth) and kept during the measurement process.
For continuous measurement of total gaseous mercury, Hg–RA-915 + ZEEMAN Mercury Analyser (Lumex Ltd.) was used as specified by Nowak et al. (2014). Measurements were repeated ten times. Air was pumped through the chamber at a constant flow rate of 5 L/min. The time of the mercury flux (single measurement) was 15 min. Calibration was performed using calibration cuvettes with high concentrations of mercury vapour in the range of 0–40.200 ng/m3. The maximum measurable concentration was about 200,000 ng/m3.
The limit of quantification for the 1 s duration of a single measurement was 500 ng/m3. The relative values of repeatability and uncertainty in percentage terms amounted to 20 %.
The one-way analysis of variance (ANOVA, Statistica 10) was used for statistical analysis. The LSD (Least Significant Difference) test was used for pairwise comparisons of means at the 0.05 level. Linear correlation was employed to analyse the relationship between the investigated parameters.
Results
Soil properties
The mercury contaminated site was tested for stabilisation and aided phytostabilisation during an outdoor plot experiment. The soil was categorised as sandy loam with an average pH of 7.39 and organic matter of 9.14 % (Table 3). The soil showed good capacity to hold calcium, magnesium and potassium. About 42 % of mercury in soil was bound to organic matter and precipitated as sulphides, below 1 % occurred in the water-soluble fraction, 16 %- in the exchangeable fraction, and 6 % in the fraction bound to humic and fulvic acids. The rest of mercury in the soil (about 35 %) was neutral for the environment. The fraction that contributed the most to bioavailability of Hg was the exchangeable fraction.
Before chemical stabilisation (Table 4) the total average soil pH was neutral (7.18). At the end of the experiment a decrease of pH values was observed with no statistical significance. In all investigated variants, excluding + sulphur variant, pH values were higher when compared to the control plots.
The EC values, analysed at the end of the experiment, were significantly higher after the addition of sulphur, however, comparable to the EC values on the planted plots.
Phytostabilisation (planted plots) and aided phytostabilisation (phyto-chemostabilisation plots with sulphur + planted) processes were observed and analysed during the experiment. Mercury plant uptake was related to its concentrations in bioavailable soil fractions. In particular water-soluble and exchangeable fractions were regarded as highly mobile and leachable, therefore the evaluation of stabilisation properties was performed based on the reduction of water-soluble and exchangeable mercury compounds. Sulphur addition to the soil resulted in about three times lower concentrations of mercury in the water-soluble soil fraction (Fig. 2). Nearly 60 % of water-soluble mercury compounds were converted into more stable compounds. The data showed that Poa pratensis did not interfere with the mercury concentration in the water-soluble soil fraction.
The stabilising effect of sulphur addition to the soil on Hg concentration in the exchangeable fraction (36 % decrease) has already been observed after chemical stabilisation in the variant with sulphur (Fig. 3). A mercury concentration decrease of 13 % in this fraction was recorded in the variant with sulphur and grass and a 22 % decrease- in planted variants with no additive. After 14 weeks of chemical stabilisation nearly 64 % of the exchangeable mercury compounds were converted into more stable compounds in variants with sulphur and 40 % in variants with sulphur and Poa pratensis. No differences between variants planted with Poa pratensis and control plots (no plants) were observed. The stabilisation effect of mercury in the presence of sulphur was about 20 % lower than in the case of plots that were planted.
During the period of geochemical soil stabilisation the lack of statistically significant differences in concentrations of mercury bound to the humic and fulvic soil fraction was observed (Fig. 4), whereas at the end of the experiment the addition of sulphur to the soil resulted in the decrease of mercury concentrations in the fulvic and humic acid fraction.
The analysed mercury contaminated soil contained, depending on the plot, about 1200–1900 kg mg−1 of HgS. At the end of the experiment, the increase of HgS concentrations in soil on plots with sulphur was recorded (Fig. 5). HgS concentration in soil was higher by about 23 % on plots with sulphur and about 38 % higher when plots were planted.
Biomass and mercury concentration
Observations of plants on the planted plots showed that mercury contaminated soil did not restrict the plants growth and development. Sulphur soil treatment increased mainly the biomass of roots (Fig. 6). It was over 50 % higher than in the case of untreated plots. Differences between the biomass of shoots produced during the growing season were not statistically significant.
Hg concentrations in plants were very high (Fig. 7) with higher accumulation in roots than in aerial parts. The comparison between roots and aerial parts of Poa pratensis showed concentration ratios of about 3.8. Sulphur addition to the contaminated soil decreased Hg concentrations in shoots by about 50 % and roots by about 30 % (Fig. 7). Depending on sulphur addition the mercury concentration ratio in the roots/shoots was about 5.7.
The correlation coefficient of Hg concentration in root/shoot was significant and positive, regardless of S additive, however in the presence of sulphur a lower r value was observed (r = 0.99 and r = 0.85 respectively; p < 0.05).
Soil leachates
The impact of rainwater on Hg concentration in leachates, as well as relations between Hg concentrations and sulphate ions depending on sulphur addition and planting were studied. In natural conditions the concentration of Hg ions during the growing season (six sampling events; Table 5) decreased after the addition of sulphur, however, the same reduction of Hg concentration was observed in the planted variant. During the same period the increase of the pH value was observed only in leachates on planted plots. Nevertheless, the value was comparable to that from the S-amended plots. Sulphur addition to the Hg contaminated soil resulted in the increased concentration of sulphate ions in leachates. The same relationship was observed for TOC concentration in leachates.
Concentration of Hg ions in the last sampling of leachates (Fig. 8) showed over four times greater release of Hg in control plots, whereas sulphur addition and planting significantly reduced the Hg concentration in relation to the control plot. A similar effect in Hg concentration was also observed in other investigated variants.
Concentration of sulphate ions in leachates showed very low and insignificant correlation to the investigated variants. As far as TOC is concerned, its concentration in leachates was significantly correlated with Hg ions, although the value of the correlation coefficient in control leachates was very low. (Table 6). No significant correlation between Hg and sulphate ion concentrations was observed in leachates, however concentrations of these ions in soil solution were negatively correlated with the increase of soil HgS concentrations, calculated as differences between sulphide concentrations at the end and at the beginning of the experiment (Fig. 9). Mercury was also significantly correlated with TOC concentration in leachates.
Soil microorganisms
The native microbial community was characterised by domination of Streptomyces and Pseudomonas, while the rest of microorganisms were at the same level (Table 7). As the plant cover influenced on soil microorganisms, significant increase of Pseudomonas, gram-negative bacteria and the number of soil fungi in rhizosphere was detected.
The dominant groups of the grass rhizosphere in the mercury contaminated soil were: Pseudomonas, gram-negative bacteria and Streptomyces. A lower share of fungi, nitrate decomposing bacteria and sulphur-amino acid decomposing bacteria in the rhizosphere was observed. Sulphur addition to the soil contributed to the increased number of nitrate and sulphur-amino acid decomposing bacteria and decrease the number of gram-negative, Pseudomonas and Streptomyces bacteria. No difference among the number of fungi in rhizosphere was observed.
Mercury evaporation and evapotranspiration
Hg evaporation was measured in the control variant and in soil with sulphur treatment, whereas evaporation and volatilisation was measured in planted variants (Fig. 10). Hg flux values in variants with sulphur treatment and with plant cover were significantly different from the control one. The flux of Hg from the planted soil and sulphur treated soil (no plants) was 81 % - 84 % lower (respectively) than in the control pots, and about 73 % lower in variants treated with sulphur and planted. The presence of Poa pratensis significantly enhanced the Hg concentration in ambient air of planted variants and in planted variants with sulphur.
Discussion
In the presented study the efficiency of using granular sulphur as stabiliser and Poa pratensis in combination as an ecological strategy for Hg-contaminated soil was evaluated. The developed strategy is based on diminishing the contaminant migration via sorption or precipitation and physical soil stabilisation by the root system, which releases nutrients that sustain a rich microbial community in the rhizosphere, enhancing the bioremediation processes. The effectiveness of these processes can be improved by a soil additive such as sulphur.
Soil properties
In accordance with Wang et al. (1982 after Patra and Sharma 2000) the permissible levels of mercury for calcareous soils was calculated to be 17 mg kg−1, while the soil used in the presented experiment contained over nineteen times higher Hg concentrations with visible drops of Hg(0) during warm days.
The elevated levels of soil Hg significantly exceeded the standard values for industrial and waste disposal sites (30 mg Hg kg−1 dry soil according to the Federal Register). The soil was categorised as sandy loam with average pH of 7.18 ± 0.36 and a high level of organic matter (9.14 %). According to Martin et al. (2009), the calcareous nature of soil helps minimise the effect of mercury load in Spanish top-soils characterised with low organic matter. According to John et al. (1975) and Frank et al. (1976) soils with high content of organic matter also have a higher average mercury content when compared to mineral soils. The investigated soil did not show any significant correlation between Hg and the organic matter content. A highly significant correlation was reported by Låg and Steinnes (1978) in the case of forest soils.
The analysis of soil from the vicinity of the chlor-alkali plant in Poland showed that about 42 % of mercury was bound to organic matter and precipitated as sulphides, below 1 % occurred in the water-soluble fraction, 17 %- in the exchangeable fraction and 6 % in the fraction bound to humic and fulvic acids. The rest of the mercury in the soil (about 35 %) was neutral to the environment. Mercury in soil exists in many forms, but Hg+2 is regarded as the predominant and readily bioavailable form (Heaton et al. 2005; Chen and Yang 2012). Mercury in water-soluble and exchangeable fractions is considered to create a negative impact on groundwater (Rodrigues et al. 2012) and to be easily accumulated by living organisms (Zagury et al. 2006; Petruzzelli et al. 2012; Carrasco-Gil et al. 2012).
Biomass of plants and mercury concentration
The investigated soil was characterised by total nitrogen, phosphorus and potassium of 0.12 %, 13.50 mg 100 g−1 (P2O5) and 12.9 mg 100 g−1 (K2O) respectively, which created the appropriate conditions for plant growth and development. The main limiting soil factor was the high Hg soil content of an average concentration of 3284 ± 1702 mg kg−1. Poa pratensis planted in experimental soil showed no visible signs of Hg toxicity stress during all the vegetation period despite very high Hg tissue concentrations (Fig. 7) in contrast with Hg induced stunted growth in wheat (Ge et al. 2009) as well as in the Dicots (Cho and Park 2000; Cargnelutti et al. 2006; Zhou et al. 2007, 2008). The reason for the successful growth of plants on such a contaminated soil may be associated with the close to neutral soil pH (7.18 ± 0,36) and high organic matter content (9.14 %) combined with the high organic carbon content (5.03 %).
Observations of Poa pratensis on the planted plots showed that mercury contaminated soil did not visually restrict the plant growth and development. Sulphur soil treatment increased significantly the biomass of roots - by over 50 % (Fig. 6) in relation to plots with no sulphur.
According to Ericksen and Gustin (2004) the investigated Poa pratensis, as a vascular plant growing on soil contaminated with Hg, uptakes this element from the soil solution (in ionic form), through the stomata from the atmosphere (in volatile form) and as divalent Hg through foliar adsorption. It is very likely that in our experiment most of the extracted Hg came via the roots from the contaminated soil, and only an insignificant quantity might have been extracted via shoots from the ambient air. Hg vapours uptake by leaves of the C3 species like Poa pratensis might be up to five times greater in relation to C4 plants (Patra and Sharma 2000).
Roots and shoots of Poa pratensis showed high Hg accumulation level in tissues; higher in roots than in aerial part of plants (about 1800 mg kg−1 and 580 mg kg−1, respectively) (Fig. 7), but lower than recorded in the soil from chlor-alkali plant in the Netherlands and Belgium (Zagury et al. 2006). The data demonstrated that Poa pratensis shoots behaved as Hg excluders, however its roots, which showed Hg includer characteristics (Patra and Sharma 2000), did not always act as an effective barrier by reducing the potential of root-to-shoot transfer of Hg, as discussed by Rodrigues et al. (2012).
Lack of an effective barrier in Hg root/shoot transport may be responsible for the recorded high concentration of Hg in shoots. The comparison between Hg concentrations in Poa pratensis roots and shoots showed the concentration ratio of about 3.2, which was in accordance with Zagury et al. (2006) observations for Hordeum vulgare. However, after the addition of sulphur the concentration ratio increased up to 4.2.
Evaluation of root and shoot Hg uptake by Poa pratensis did not show any statistical significance, although positive correlation was reported by Suszcynsky and Shann (1995); Patra and Sharma (2000); Heaton et al. (2003) and Greger et al. (2005).
A significant relationship between levels of Hg in the soil and those in roots and shoots of the investigated grasses reported by Rodrigues et al. (2012) was not observed on our plots. Evaluation of Hg transport from the contaminated soil to plants and animals indicated that for ryegrass and orchard grass the Hg levels were controlled not only by the total concentration of Hg in soil but also by the levels of Alox and Feox (Rodrigues et al. 2012).
Soil leachates
The volume of leachates collected during the growing season varied irrespective of the investigated variants. The high content of soil organic matter was suspected to be the cause for the lack of significant impact of the plant cover on leachate volume. The environmental risk of leachates in relation to EU standards for drinking water was related only to Hg concentrations (Table 5) exceeding the standards from 15.5 (control; no plants and no amendment) to 35.7 times (sulphur addition; no plants). Hg concentrations in the collected rain percolation waters were significantly lower than in the control plots and the same was observed in the investigated variants (Table 5). During the end of the experiment Hg ion concentrations in leachates (Fig. 8) showed over four times greater release of Hg in control plots, whereas sulphur addition as well as planting reduced significantly the Hg content in relation to the control plots. Moreover, the control soil was characterised with the Hg flux four to six times greater (Fig. 10) then the compared planted soil and sulphur treated soil (no plants), which suggests differences in the Hg biogeochemical cycle related to soil properties and the soil/plant relationship. According to the Conceptual Model for soil mercury flux (Briggs and Gustin 2013), if the soil becomes saturated, Hg flux will be suppressed due to the soil pores becoming filled with water. In the soil profile Hg desorption and dilution processes dominate. Johnson et al. (2003) states that the available Hg (II) will dissolve into the soil water and Hg0 from soil particles will be desorbed into soil gas. The enhanced release of mercury from highly contaminated soil during precipitation events observed in the end of the vegetation period may be similar to the processes observed by Lindberg et al. (1999) on a dry desert soil, i.e.: physical displacement of Hg(0) soil gas by water filling the soil pores, replacement of Hg(0) adsorbed to the soil water molecules, desorption of Hg(II) bound to the soil particles, reduction to Hg(0) through abiotic and biotic factors or photosolubilisation of soil cinnabar leading to generation of soil Hg(II). An increase of SO4 − ions and TOC concentrations was noticed after the addition of sulphur to the soil. Leachate sulphate ions were significantly negatively correlated with soil HgS concentrations, calculated as differences between soil sulphide concentration content at the end and at the beginning of the experiment (Fig. 9). Concentration of Hg in leachates was significantly correlated with TOC concentration, especially after sulphur addition and planting, however a very low correlation coefficient was observed in control plots. On plots amended with granular sulphur the increase of soil sulphides resulted in the decrease of sulphate ions in leachates. The process can be explained by oxidative conditions created by the presence of plants (Pennington and Walters 2006).
Soil microorganisms
The soil microflora plays a vital role in soil fertility, organic matter decomposition and nutrient cycling. The presence of sulphur in mercury contaminated soil does not affect the total number of bacteria and the number of gram-negative bacteria in rhizosphere. Presence of Pseudomonas (which caused an unwanted methylation process) and the number of soil fungi were at the same level of bacterial count, irrespective of sulphur addition into the soil environment. After sulphur addition a significantly higher number of nitrate decomposing bacteria and sulphur-amino acid decomposing bacteria was noted. The increased number of nitrate decomposing bacteria (Table 6) can result in possible faster conversion of Hg soil nitrates into atmospheric nitrogen.
The number of sulphur-amino acid decomposing bacteria was found to be in an inverse correlation with root mercury uptake in soil from the chlor-alkali plant (Sas-Nowosielska et al. 2008) which was explained as a positive bioremediation effect on soil mercury. The obtained results (Table 7) showed a positive effect on sulphur-amino acid decomposing bacteria after addition of granular sulphur to the Hg contaminated soil.
Mercury evaporation and evapotranspiration
According to Schlüter (2000) the Hg evaporation occurs in the uppermost soil layers. Presented data indicate that soil from the chlor-alkali plant evaporates high levels of Hg to the atmosphere and highlight the importance of sulphur additive to the soil as well as creation of the plant cover in reducing the Hg flux to about 70 % - 80 % in relation to unplanted plots (Fig. 8). A very high level of Hg concentrations in the atmosphere in the chlor-alkali area was also confirmed by Esbrí et al. (2014).
Mercury as a soil contaminant may evaporate into the air in both organic and elemental form. In our experiment (Fig. 10) the flux of Hg from the planted soil and sulphur treated soil (no plants) was 81 % and 84 % lower (respectively) than in the control pots, and about 73 % lower in variants treated with sulphur and planted. According to literature (Kocman and Horvat 2010) Hg volatilisation occurs more often in sites where mobile Hg forms are dominant than in sites where insoluble cinnabar prevails. The decreased level of Hg in the flux after sulphur application (Fig. 10) may be attributed to the formation of insoluble HgS. The decrease in Hg flux observed in planted soil variants (Poa pratensis) is most probably due to the physical barrier formed by the dense plant cover as well as due to the influence of Hg on stomata opening. Hg2+ ions in leaf tissues can bind to aquaporins (water channel proteins) inducing the closure of stomata (Zhang and Tyerman 1999). This, in turn, may inhibit the mercury evapotranspiration process.
In the variant with sulphur and P. pratensis the flux of Hg was higher than in the variant with sulphur (+ sulphur) and the variant with Poa pratensis (planted). The observed Hg concentration in flux may be related to the soil formed SO2 as highlighted by Macdonald et al. (2004). They show that soil can be the source of sulphur dioxide (SO2) which evaporates into the atmosphere. The atmospheric SO2 may enhance opening of stomata in herbaceous plants (Majernik and Mansfield 1970; Majernik 1971; Unsworth et al. 1972; Biscoe et al. 1973; Noland and Kozlowski 1979). SO2 enters the stomatal complex via adjacent epidermal cells and at low SO2 concentrations the turgor in these cells is reduced, which induces stomatal opening (Black and Unsworth 1980). This, in turn, may generate the observed increase in Hg flux.
Remediation aspect
An ecological strategy for soil contaminated with mercury was analysed. Aided stabilisation, phytostabilisation and aided phytostabilisation were tested during one-year experiment as a remedial option for mercury-contaminated soil.
According to the obtained data the investigated soil was rich with organic matter, had good ability to hold the fertilizing ions e.g. nitrogen, phosphate and potassium and supported plant growth and development. The soil was amended with sulphur and planted for Hg stabilisation. According to Luo et al. (2009) soil organic matter, nitrogen and sulphur contributed to Hg retention in soil, forming effective Hg soil mineral adsorbents, which was also observed by Barnett et al. (1997). The increase of soil sulphide concentrations after granular sulphur addition, especially in planted variants showed plant rhizosphere participation in sulphide concentration. Mercury can be bound very tightly to sulphur forming the insoluble HgS, which was noted also by Boszke et al. (2008). According to Bloom and Katon (2000) the contribution of mercury bound to sulphides in the mercury-contaminated soil was 99.1 % in the vicinity of the plant producing chlorine alkaline compounds. Similarly, most of Hg in the investigated soil (about 77 %) was precipitated as sulphides (1379.28 ± 9.58 mg kg−1) or bound to either organic matter or residual soil fraction (1150.08 ± 10.12 mg kg−1), meaning it was less mobile (Table 3). At the end of the presented experiment, the increase of HgS concentrations in soil on planted plots with sulphur was recorded (Fig. 5). According to investigation on solubility and distribution/transformation of mercury in the solid-phase components of soil contaminated with various forms of mercury, the plants effects on mercury solid-phase components were dependent upon mercury sources and loading levels. The presence of plants decreased mercury concentrations in the mercury bound to crystalline iron oxides extracts fraction and increased mercury in the residual fractions (Han et al. 2006).
The mercury-contaminated soil stabilisation effect was also supported by the presence of sulphur-amino acid decomposing bacteria which was found to be in an inverse correlation with root mercury uptake (Sas-Nowosielska et al. 2008). The observed increase of sulphur-amino acid decomposing bacteria in the rhizosphere (Table 7) in the presence of sulphur may be considered as biostabilisation of mercury in contaminated soil.
The evaluation of remediation processes on Hg contaminated soils requires consideration of the potential destabilisation of HgS compounds when soil conditions become aerobic due to the decreased content of the organic matter or seasonal turnover which leads to sulphide oxidization to sulphate and releasing the mercury in the ionic form Hg(II), susceptible to the methylation process as was observed by Ullrich et al. (2001) and Boszke et al. (2003). The X-ray absorption fine structure spectroscopic studies (Skyllberg et al. 2006) revealed that the reduced organic sulphur and oxygen/nitrogen groups were involved in the complexation of Hg (II) to humic substances extracted from organic soils. According to Maclean (1974) the clay-sand soils released considerable amounts Hg to the CaCl2 solution while no Hg was detected in the extract of the corresponding S-treated samples. Direct interaction between Hg and S led to the formation of metacinnabar or cinnabar (Outridge et al. 2001). Bloom and Katon (2000) showed that metacinnabar solubility in soil was extremely low, even when compared to the soil matrix minerals. Moreover, the obtained results indicated that in the optimization of the Hg contaminated soil remediation efforts we should take into consideration that SO2 formed by the addition of sulphur additive may enhance the stomata opening, increasing the Hg flux to the atmosphere and affecting the soil/plant/atmosphere Hg- cycle.
Creation of the plant cover requires supplementary addition of nitrogen fertilisers. In the application of nitrate fertilisers to the Hg contaminated soil it should be noted that higher concentrations of NO3 − ions in the soil may result in an increased Hg plant uptake as was observed by Carrasco-Gil et al. (2012) in alfalfa plants. The presence of sulphur in the Hg contaminated soil increased the number of nitrate decomposing bacteria (Table 6), which can be explained by faster conversion of nitrates into atmospheric nitrogen and reduction of risk connected with presence of NO3 − ions in soil. The plants grown on mercury-contaminated soil collected from the chlor-alkali plant developed a rhizosphere area rich in microorganisms (Sas-Nowosielska et al. 2008). At the same time plant roots appeared to be mercury includers. Both formation of the plant cover and application of soil amendments are essential for soil stabilisation used in phytoremediation technologies.
References
Alef K (1995) Enrichment, isolation and counting of soil microrganisms. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp. 123–186
Asztalos E, Sipka G, Kis M, Trotta M, Maróti P (2012) The reaction center is the sensitive target of the mercury (II) ion in intact cells of photosynthetic bacteria. Photosynth Res 112(2):129–140
Barillot CDC, Sarde CO, Bert V, Tarnaud E, Cochet N (2012) A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann Microbiol 63(2):471–476
Barnett MO, Harris LA, Turner RR, et al. (1997) Formation of mercuric sulphide in soil. Environ Sci Technol 31(11):3037–3043
Biscoe PV, Unsworth MH, Pinckney HR (1973) The effects of low concentrations of Sulphur dioxide on stomatal behaviour in Vicia faba. New Phytol 72:1299–1306
Black VJ, Unsworth MH (1980) Stomatal responses to sulphur dioxide and vapour pressure deficit. J Exp Bot 31(2):667–677
Bloom NS, Katon J (2000) Application of selective extractions to the determination of mercury speciation in mine tailings and adjacent soils. In: Proceeding of assessing and managing mercury from historic and current mining activities conference, San Francisco. http://www.researchgate.net/publication/228479568_Application_of_selective_extractions_to_the_determination_of_mercury_speciation_in_mine_tailings_and_adjacent_soils. Accessed 28 June 2016
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Boszke L, Kowalski A, Głosińska G, Szarek R, Siepak J (2003) Environmental factors affecting speciation of mercury in the bottom sediments; an overview. Pol J Environ Stud 12:5–13
Boszke L, Kowalski A, Astel A, Barański A, Gworek B, Siepak J (2008) Mercury mobility and bioavailability in soil from contaminated area. Environ Geol 55:1075–1087
Bower J, Savage KS, Weinman B, Barnett MO, Hamilton WP, Harper WF (2008) Immobilization of mercury by pyrite (FeS2). Environ Pollut 156:504–514
Briggs C, Gustin MS (2013) Building upon the conceptual model for soil mercury flux: evidence of a link between moisture evaporation and Hg evasion. Water Air Soil Pollut 224(1744):1–13
Cachada A, Rodrigues SM, Mieiro C, Ferreira da Silva E, Pereira E, Duarte AC (2009) Controlling factors and environmental implications of mercury contamination in urban and agricultural soils under a long term influence of a chlor-alkali plant in north-West Portugal. Environ Geol 57:91–98
Cargnelutti D, Tabaldi LA, Spanevello RM, de Oliveira JG, Battisti V, Redin M, Linares CEB, Dressler VL, de Moraes Flores EM, Nicoloso FT, Morsch VM, Schetinger MRC (2006) Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere 65:999–1006
Carrasco-Gil S, Estebaranz-Yubero M, Medel-Cuesta D, Millan R, Hernandez LE (2012) Influence of nitrate fertilisation on Hg uptake and oxidative stress parameters in alfalfa plants cultivated in a Hg-polluted soil. Environ Exp Bot 75:16–24
Chen J, Yang ZM (2012) Mercury toxicity, molecular response and tolerance in higher plants. Biometals 25:847–857
Cho UH, Park JO (2000) Mercury-induced oxidative stress in tomato seedlings. Plant Sci 156:1–9
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10:471–477
Di Giulio RT, Ryan EA (1987) Mercury in soils, sediments and clams from a North Carolina peatland. Water Air Soil Pollut 33:205–219
Dufault R, LeBlanc B, Schnoll R, Cornett C, Schweitzer L, Wallinga D, Hightower J, Patrick L, Lukiw WJ (2009) Mercury from chlor-alkali plants: measured concentrations in food product sugar. Environ Health 8:1–6
Ericksen JA, Gustin MS (2004) Foliar exchange of mercury as a function of soil and air mercury concentrations. Sci Total Environ 324:271–279
Esbrí JM, López-Berdonces MA, Fernández-Calderón S, Higueras P, Díez S (2014) Mercury atmospheric pollution around a chlor-alkali plant in Flix (NE Spain): an integrated analysis. Environ Sci Pollut Res 22:4842–4850
Ferrara R, Mazzolai B (1998) A dynamic flux chamber to measure mercury emission from aquatic systems. Sci Total Environ 215:51–57
Frank R, Ishida K, Suda P (1976) Metals in agricultural soils of Ontario. Can J Soil Sci 56:181–196
Fuhrmann M, Melamed D, Kalb PD, Adams JW, Milian LW (2002) Sulphur polymer solidification/stabilisation of elemental mercury waste. Waste Manag 22:327–333
Ge C, Ding Y, Wang Z, Wan D, Wang Y, Shang Q, Luo S (2009) Responses of wheat seedlings to cadmium, mercury and trichlorobenzene stresses. J Environ Sci 21:806–813
Grant MA, Holt JG (1977) Medium for the selective isolation of members of the genus Pseudomonas from natural habitats. Appl Environ Microbiol 33:1222–1224
Greger M, Wang Y, Neuschütz C (2005) Absence of Hg transpiration by shoot after Hg uptake by roots of six terrestrial plant species. Environ Pollut 134:201–208
Han FX, Su Y, Monts DL, Waggoner CA, Plodinec MJ (2006) Binding, distribution and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Sci Total Environ 368:753–768
Heaton ACP, Rugh CL, Kim T, Wang NJ, Meagher RB (2003) Toward detoxifying mercury-polluted aquatic sediments with rice genetically engineered for mercury resistance. Environ Toxicol Chem 22:2940–2947
Heaton ACP, Rugh CL, Wang NJ, Meagher RB (2005) Physiological responses of transgenic mer A-tobacco (Nicotiana tabacum) to foliar and root mercury exposure. Water Air Soil Pollut 161:137–155
Hobman JL, Brown NL (1997) Bacterial mercury-resistance genes. In: Sigel A, Sigel H (eds) Metal ions in biological systems, volume 34 mercury and its effects on environment and biology. Marcel Dekker INC, New York, pp. 527–560
Hooda PS (2010) Trace elements in soils. Blackwell Publishing Ltd
John MK, Van Laerhoven CJ, Osborne VE, Cotic I (1975) Mercury in soils of British Columbia, a mercuriferous region. Water Air Soil Pollut 5:213–220
Johnson DW, Benesch JA, Gustin MS, Schorran DS, Lindberg SE, Coleman JS (2003) Experimental evidence against diffusion control of Hg evasion from soils. Sci Total Environ 304:175–184
Kocman D, Horvat M (2010) A loboratory based experimental study of mercury emission from contaminated soils in the river Idrijca catchment. Atmos Chem Phys 10:1417–1426
Låg J, Steinnes E (1978) Regional distribution of mercury in humus layers of Norwegian forest soils. Acta Agric Scand 28:393–396
Lagerwerff JV (1967) Heavy-metal contamination of soils. In: Agriculture and the quality of the environment. American Association for the Advancement of Science Publication No. 85, Washington D.C. pp 343–364
Lindberg SE, Zhang H, Gustin M, Vette A, Marsik F, Owens J, Casimir A, Ebinghaus R, Edwards G, Fitzgerald C, Kemp J, Kock HH, London J, Majewski M, Poissant L, Pilote M, Rasmussen P, Schaedlich F, Schneeberger D, Sommar J, Turner R, Wallschläger D, Xiao Z (1999) Increases in mercury emissions from desert soils in response to rainfall and irrigation. J Geophys Res 104(21):879–21,888
Luo W, Lu Y, Wang B, Tong X, Wang G, Shi Y, Wang T, Giesy JP (2009) Distribution and sources of mercury in soils from former industrialized urban areas of Beijing, China. Environ Monit Assess 158:507–517
Macdonald BCT, Denmead OT, White I, Melville MD (2004) Natural sulfur dioxide emissions from sulfiric soils. Atmos Environ 38:1473–1480
Maclean AJ (1974) Mercury in plants and retention of mercury by soils in relation to properties and added sulfur. Can J Soil Sci 54:287–292
Majernik O (1971) A physiological study of the effects of SO2 pollution, phenylmercuric acetate sprays, and parasitic infection on stomatal behaviour and ageing in barley. J Phytopathol 72:255–268
Majernik O, Mansfield TA (1970) Direct effect of SO2 pollution on the degree of opening of stomata. Nature 227:377–378
Martin JAR, de la Cueva AV, Corbi JMG, Alonso CM, Arias ML (2009) Factors controlling the spatial variability of mercury distribution in Spanish topsoil. Soil Sediment Contam Int J 18:30–42
Noland TL, Kozlowski TT (1979) Effect of SO2 on stomatal aperture and Sulphur uptake of woody angiosperm seedlings. Can J For Res 9:57–62
Nowak B, Korszun K, Zielonka U (2014) Long-term measurements of atmospheric mercury species (TGM, TPM) and Hg deposition in the Silesian region, Poland – conception of the mercury deposition coefficient. Arch Environ Prot 40:43–60
Outridge P, Percival JB, Lockhart WL, Stern G (2001) Geochemical and mineralogical investigations of potential chemical diagenesis in a varved Arctic lake sediment. Proceedings of the 6th international conference biogeochemistry of trace elements. University of Guelph, Canada, p. 69
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422
Pennington MR, Walters MB (2006) The response of planted trees to vegetation zonation and soil redox potential in created wetlands. For Ecol Manag 233:1–10
Pereira ME, Lillebø AI, Pato P, Válega M, Coelho JP, Lopes CB, Rodrigues SM, Cachada A, Otero M, Pardal MA, Duarte AC (2009) Mercury pollution in Ria de Aveiro (Portugal): a review of the system assessment. Environ Monit Assess 155:39–49
Petruzzelli G, Pedron F, Barbafieri M, Tassi E, Gorini F, Rosellini I (2012) Enhanced bioavailable contaminant stripping: a case study of Hg contaminated soil. Chem Eng Trans 28:211–216
Piao H, Bishop PL (2006) Stabilization of mercury-containing wastes using sulphide. Environ Pollut 139:498–506
Randall PM, Chattopadhyay S (2013) Mercury contaminated sediment sites-an evaluation of remedial options. Environ Res 125:131–149
Rodrigues SM, Henriques B, Reis AT, Duarte AC, Pereira E, Römkens PFAM (2012) Hg transfer from contaminated soils to plants and animals. Environ Chem Lett 10:61–67
Rodriguez L, Lopez-Bellido FJ, Carnicer A, Recreo F, Tallos A, Monteagudo JM (2005) Mercury recovery from soils by phytoremediation. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) Environmental chemistry. Green chemistry and pollutants in ecosystems. Springer-Verlag, Berlin Heidelberg, pp. 197–204
Sas-Nowosielska A, Galimska-Stypa R, Kucharski R, Zielonka U, Małkowski E, Gray L (2008) Remediation aspect of microbial changes of plant rhizosphere in mercury contaminated soil. Environ Monit Assess 137:101–109
Schlüter K (2000) Review: evaporation of mercury from soils. An integration and synthesis of current knowledge. Environ Geol 39:249–271
Schwesig D, Ilgen G, Matzner E (1999) Mercury and methylmercury in upland and wetland acid forest soils of a watershed in NE-Bavaria, Germany. Water Air Soil Pollut 113:141–154
Skyllberg U, Bloom PR, Qian J, Lin CHM, Bleam WF (2006) Complexation of mercury (II) in soil organic matter: EXAFS evidence for linear two-coordination with reduced sulfur groups. Environ Sci Technol 40:4174–4180
Suszcynsky EM, Shann JR (1995) Phytotoxicity and accumulation of mercury in tobacco subjected to different exposure routes. Environ Toxicol Chem 14:61–67
Ullrich SM, Tanton TW, Abdrashitowa SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
Unsworth MH, Biscoe PV, Pinckney HR (1972) Stomatal responses to Sulphur dioxide. Nature 239:458–459
Wallschläger D, Desai MVM, Spengler M, Wilken RD (1998) Mercury speciation in floodplain soils and sediments along a contaminated river transect. J Environ Qual 27:1034–1044
Wang J, Feng X, Anderson CWN, Xing Y, Shang L (2012) Remediation of mercury contaminated sites – a review. J Hazard Mater 221-222:1–18
Zagury GJ, Neculita CM, Bastien C, Deschênes L (2006) Mercury fractionation, bioavailability and ecotoxicity in highly contaminated soils from chlor-alkali plants. Environ Toxicol Chem 25:1138–1147
Zhang WH, Tyerman SD (1999) Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol 120:849–858
Zhang XY, Wang QC, Zhang SQ, Sun XJ, Zhang ZS (2009) Stabilisation/solidification (S/S) of mercury-contaminated hazardous wastes using thiol-functionalized zeolite and Portland cement. J Hazard Mater 168:1575–1580
Zhou ZS, Huang SQ, Guo K, Mehta SK, Zhang PC, Yang ZM (2007) Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J Inorg Biochem 101:1–9
Zhou ZS, Wang SJ, Yang ZM (2008) Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 70:1500–1509
Zuo XJ, Fu DF, Li H (2013) Variation characteristics of mercury in speciation during road runoff for different rainfall patterns. CLEAN-Soil, Air, Water 41:69–73
Acknowledgments
The study was carried out under the grant NN523 750 340 from the Polish National Science Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pogrzeba, M., Ciszek, D., Galimska-Stypa, R. et al. Ecological strategy for soil contaminated with mercury. Plant Soil 409, 371–387 (2016). https://doi.org/10.1007/s11104-016-2936-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2936-8