Abstract
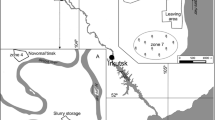
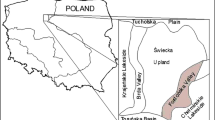
The mobility and bioavailability of mercury in the soil from the area near a plant using elemental mercury for manufacturing thermometers, areometers, glass energy switches and other articles made of technical glass has been evaluated. Mercury has been determined by sequential extraction method and with additional thermo desorption stage to determine elemental mercury. The procedure of sequential extraction involves five subsequent stages performed with the solutions of chloroform, deionized water, 0.5 M HCl, 0.2 M NaOH and aqua regia. The mean concentration of total mercury in soil was 147 ± 107 μg g−1 dry mass (range 62–393), and the fractionation revealed that mercury was mainly bound to sulfides 56 ± 8% (range 45–66), one of the most biounavailable and immobile species of mercury in the environment. The fractions that brought lower contribution to the total mercury content were semi-mobile humic matter 22 ± 9% (range 11–34) and elemental mercury 17 ± 5% (range 8–23). The contributions brought by the highly mobile and toxic organomercury compounds were still lower 2.3 ± 2.7% (range 0.01–6.5). The lowest contributions brought the acid-soluble mercury 1.5 ± 1.3% (range 0.1–3.5) and water-soluble mercury 1.0 ± 0.3% (range 0.6–1.7). The surface layer of soil (0–20 cm) was characterized by higher mercury concentrations than that of the subsurface soil (60–80 cm), but the fractional contributions were comparable. The comparison of mercury fractionation results obtained in this study for highly polluted soils with results of fractionation of uncontaminated or moderately contaminated samples of soil and sediments had not shown significant statistical differences; however, in the last samples elemental mercury is usually present at very low concentrations. On the basis of obtained correlation coefficients it seems that elemental mercury soils from “Areometer” plant are contaminated; the main transformation is its vaporization to atmosphere and oxidation to divalent mercury, probably mainly mediated by organic matter, and next bound to humic matter and sulfides.




Similar content being viewed by others
References
Barnett MO, Turner RR (2001) Bioaccessibility of mercury in soils. Soil Sediment Contam 10:301–316
Bełdowski J, Pempkowiak J (2003) Horizontal and vertical variabilities of mercury concentration and speciation in sediments of Gdańsk Basin, Southern Baltic Sea. Chemosphere 52:645–654
Biester H, Scholz C (1997) Determination of mercury phase in contaminated soils. Mercury pyrolysis versus sequential extractions. Environ Sci Technol 31:233–239
Biester H, Müller G, Schöler HF (2002) Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Sci Total Environ 284:191–203
Bloom NS, Katon J (2000) Application of selective extractions to the determination of mercury speciation in mine tailings and adjacent soils. In: Proceeding of assessing and managing mercury from historic and current mining activities conference, San Francisco, 28–30 November 2000
Bloom NS, Preus E, Katon J, Hilter M (2003) Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediment and soils. Anal Chim Acta 479:233–248
Bobrowicz M (1999) Measurement of mercury concentration in soil samples within the area of “Areometer” Plant. PPH Biomed, Warsaw (in Polish)
Boszke L, Astel A (2007) Fractionation of mercury in sediments from coastal zone inundated by tsunami and in freshwater sediments from rivers. J Environ Sci Health A42:847–858
Boszke L, Kowalski A (2006) Spatial distribution of mercury in bottom sediments and soils from Poznań City, Poland. Polish J Environ Stud 15:211–218
Boszke L, Kowalski A (2007a) Mercury fractionation in sediments of the lower Vistula river (Poland). Oceanol Hydrobiol Stud 36:79–99
Boszke L, Kowalski L (2007b) Mercury fractionation in floodplain soils of the Warta river, Poland. Oceanol Hydrobiol Stud (in press)
Boszke L, Głosińska G, Siepak J (2002) Some aspects of speciation of mercury in a water environment. Polish J Environ Stud 11:285–298
Boszke L, Kowalski A, Głosińska G, Szarek R, Siepak J (2003) Selected factors affecting the speciation of mercury in the bottom sediments: an overview. Polish J Environ Stud 12:5–13
Boszke L, Kowalski A, Szczuciński W, Rachlewicz G, Lorenc J, Siepak J (2006) Assessment of mercury availability by fractionation method in sediments from coastal zone inundated by the 26 December 2004 tsunami in Thailand. Environ Geol 51:527–536
Boszke L, Kowalski A, Siepak J (2007) Fractionation of mercury in sediments of the Warta river (Poland). In: Pawłowski L, Dudzińska M, Pawłowski A (eds) Environmental engineering science. Taylor & Francis, London, pp 403–413
Ching IL, Hongxiao T (1985) Chemical studies of aquatic pollution by heavy metals in China. In: Irgolic KJ, Martel AE (eds) Environmental inorganic chemistry. VCH, Deerfield Beach, pp 359–371
Eguchi T, Tomiyasu T (2002) The speciation of mercury in sediments from Kagoshima Bay and Minamata Bay, southern Kyusyu, Japan, by fractional extraction/cold-vapor AAS. Bunseki Kagaku 51:859–864 (in Japanese)
Falandysz J (2002) Mercury in mushrooms and soil of the Tarnobrzeska Plain, South-Eastern Poland. J Environ Sci Health A37:343–352
Falandysz J, Kawano M, Danisiewicz D, Chwir A, Boszke L, Gołębiowski M, Boryło A (1996) Investigation on the occurrence of mercury in soils in Poland. Bromat Chem Toksykol 29:177–181 (in Polish with English abstract)
Falandysz J, Bielawski L, Kannan K, Gucia M, Lipka K, Brzostowski A (2002) Mercury in wild mushrooms and underlying soil substrate from the great lakes land in Poland. J Environ Monit 4:473–476
Falandysz J, Jędrusiak A, Lipka K, Kannan K, Kawano M, Gucia M, Brzostowski A, Dadej M (2004) Mercury in wild mushrooms and underlying soil substrate from Koszalin, North-central Poland. Chemosphere 54:461–466
Hesterberg D, Chou JW, Hutchison KJ, Sayers DE (2001) Bonding of Hg(II) to reduced organic sulfur in humic acid as affected by S/Hg ratio. Environ Sci Technol 35:2741–2745
Inacio MM, Pereira V, Pinto MS (1998) Mercury contamination in sandy soils surrounding an industrial emission source (Estarreja, Portugal). Geoderma 85:325–339
Konieczka P, Namieśnik J, Zygmunt B, Bulska E, Świtaj-Zawadka A, Naganowska A, Kremer E, Rompa M (2004) Quality assessment and quality control of analytical results—QA/QC. Centre of Excellence in Environment Analysis and Monitoring, Gdańsk (in Polish)
Kot FS, Matyushkina LA (2002) Distribution of mercury in chemical fraction of contaminated urban soils of Middle Amur, Russia. J Environ Monit 4:803–908
Kot FS, Matyushkina LA, Nikorych VN, Polivichenko VG (2002) Mercury in chemical and thermal fraction of soils of Eastern Ukrainian Polissya. Soil Sci 3:94–101
Kowalski A (2006) Determination of the total mercury and its species in the environmental samples. Doctoral Thesis, Adam Mickiewicz University, Poznań (in Polish)
Lechler PJ, Miller JR, Hsu LC, Desilets MO (1997) Mercury mobility at the Carson River superfund site, west-central Nevada, USA-interpretation of mercury speciation data in mill tailing, soils, and sediments. J Geochem Explor 58:259–267
Lityński T, Jakubowska H, Gorlach E (1976) The chemical–agricultural analysis. PWN, Warszawa (in Polish)
Miller EL, Dobb DE, Heithmar EM (1995) Speciation of mercury in soils by sequential extraction. Presented at the USEPA Metal Speciation and Contamination of Surface Water Workshop, Jekyll Island
Ostrowska A, Gawliński S, Szczubiałka Z (1991) Methods of analysis and evaluation of soil properties, catalogue. Wydawnictwo Instytutu Ochrony Środowiska, Warszawa (in Polish)
Peng A, Wang Z (1985) Mercury in river sediment. In: Irgolic KJ, Martel AE (eds) Environmental inorganic chemistry. VCH, Deerfield Beach, pp 393–400
Pestana MHD, Formoso MLL, Lechler PJ (2000) Mercury and cupper contamination from historic and recent mining activities in the Camaqua River Basin, South Brazil. In: Nriagu JO (ed) 11th Annual international conference on heavy metals in the environment. University of Michigan, School of Public Health, Ann Arbor (CD-ROM, Contribution Number:1115)
PN-ISO 10694 (2002) Soil quality—determination of organic and total carbon after dry combustion (elementary analysis) (in Polish)
Polubiec E, Bruszewski H, Kostrzewa-Szulc J, Sienkiewicz J (2003) The study on the incorporation of mercury to the biological cycle. Institute of Environmental Protection, Warsaw (in Polish)
Ravichandran M (2004) Interactions between mercury and dissolved organic matter––a review. Chemosphere 55:319–331
Renneberg AJ, Dudas MJ (2001) Transformations of elemental mercury to inorganic and organic forms in mercury and hydrocarbon co-contaminated soils. Chemosphere 45:1103–1109
Sakamoto H, Tomiyasu T, Yonehara N (1995) The content and chemical forms of mercury in sediments from Kagoshima Bay, in comparison with Minamata Bay and Yatsushiro Sea, Southwestern Japan. Geochem J 29:97–105
Shi JB, Liang LN, Jiang GB, Jin XL (2005) The speciation and bioavailability of mercury in sediments of Haihe River, China. Environ Int 31:357–365
StatSoft, Inc (2001) STATISTICA—data analysis software system, version 6. http://www.statsoft.com
Stein ED, Cohen Y, Winer AM (1996) Environmental distribution and transformation of mercury compounds. Crit Rev Environ Sci Technol 26:1–43
Ullrich SM, Tanton TW, Abdrashitowa SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
Umińska R (1987) Evaluation of mercury level in waters, bottom sediments and soils in selected rural areas. J Hyg Epidemiol Microbiol Immunol (Prague) 31:9–22
Wallschläger D, Desai MVM, Wilken R-D (1996) The role of humic substances in the aqueous mobilization of mercury from contaminated floodplain soils. Water Air Soil Pollut 90:507–520
Wallschläger D, Desai MVM, Spengler M, Windmöler CC, Wilken R-D (1998) Mercury speciation in floodplain soils and sediments along a contaminated river transect. J Environ Qual 27:1034–1044
Wang D, Shi X, Wie S (2003) Accumulation and transformation of atmospheric mercury in soil. Sci Total Environ 304:209–214
Waples JS, Nagy KL, Aiken GR, Ryan JN (2005) Dissolution of cinnabar (HgS) in the presence of natural organic matter. Geochim Cosmochim Acta 69:1575–1588
Wasay SA, Barrington S, Tokunaga S (1998) Retention form of heavy metals in three polluted soils. Soil Sediment Contam 7:103–119
Xia K, Skyllberg UL, Bleam WF, Bloom RP, Nater EA, Helmke PA (1999) X-ray absorption spectroscopic evidence for the complexation of Hg(II) by reduced sulfur in soil humic substances. Environ Sci Technol 33:257–261
Acknowledgment
Kind assistance and helpful critical remarks of prof. dr hab. Jerzy Falandysz are gratefully acknowledged. This work was partly supported by the Polish Committee for Scientific Research (KBN) under grant No 3 T09D 079 26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boszke, L., Kowalski, A., Astel, A. et al. Mercury mobility and bioavailability in soil from contaminated area. Environ Geol 55, 1075–1087 (2008). https://doi.org/10.1007/s00254-007-1056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1056-4