Abstract
Background
Bacterivores, mostly represented by protists and nematodes, are a key component of soil biodiversity involved in soil fertility and plant productivity. In the current context of global change and soil biodiversity erosion, it becomes urgent to suitably recognize and quantify their ecological importance in ecosystem functioning.
Scope
Using meta-analysis tools, we aimed at providing a quantitative synthesis of the ecological importance of soil bacterivores on ecosystem functions. We also intended to produce an overview of the ecological factors that are expected to drive the magnitude of bacterivore effects on ecosystem functions.
Conclusions
Bacterivores in soil contributed significantly to numerous key ecosystem functions. We propose a new theoretical framework based on ecological stoichiometry stressing the role of C:N:P ratios in soil, microbial and plant biomass as important parameters driving bacterivore-effects on soil N and P availability for plants, immobilization of N and P in the bacterial biomass, and plant responses in nutrition and growth.
Similar content being viewed by others
Introduction
Soils are some of the most biologically diverse environments, encompassing about 25 % of global biodiversity in terms of species (Coleman and Wall 2015; Decaëns 2010). One gram of soil can host 6000 different bacterial genomes, several meters of fungal hyphae and a wide range of protists, nematodes, enchytraeids or mites (Jones et al. 2009; Lavelle and Spain 2001; Wall et al. 2010). Through their activities, soil organisms ensure key soil functions (Barrios 2007; Brussaard et al. 2007; Wagg et al. 2014) and contribute to many ecosystem services (de Vries et al. 2013; Kibblewhite et al. 2008).
Unfortunately, soils are also among the most threatened environments in terms of biodiversity loss (Kibblewhite 2012). Many threats (soil erosion, land use change, overexploitation, pollution, biological invasion, etc.) have been identified as directly disturbing soil organism abundance, distribution and activity (Bossio et al. 2005; Dupouey et al. 2002; Foley et al. 2005; Gardi et al. 2013; Pimentel 2006; Schlaghamersky et al. 2014). A thorough understanding of the many roles of soil biodiversity is required in order to predict how these threats could damage ecosystem services accomplished by soil organisms.
A key component of soil biodiversity involved in soil fertility and plant productivity are bacterivores (Bardgett et al. 1999; Bonkowski 2004; Bonkowski et al. 2009; Brussaard 1997). These bacterivores are mostly represented by protists and nematodes (Rønn et al. 2012). Bacterivorous soil protists are generally unicellular heterotrophic eukaryotes, that were traditionally grouped into ciliates, flagellates, naked amoebae and testate amoebae (Darbyshire 1994). However, recent molecular studies reveal an enormous taxonomic diversity in soils (Geisen et al. 2014; Geisen et al. 2015), and only the ciliated protists are monophyletic, while flagellated, and naked, and testate amoeboid morphotypes repeatedly originated during protist evolution (Adl et al. 2005; Adl et al. 2012).
Despite their small size (average size 2–50 μm), protists can largely contribute to total soil faunal biomass (Bonkowski 2004). Nematodes are small multicellular eukaryotes (0.03-1 mm size range) and form a highly diverse monophyletic group including about one million species (Lambshead 1993), making this phylum one of the most diverse animal taxa on Earth (Lambshead et al. 2004). Both bacterial-feeding protists and nematodes are widely distributed in terrestrial ecosystems and successful colonizers of different soil niches (Cowling 1994; Geisen et al. 2014). It has been estimated than tens of millions of protists and hundreds of thousands of nematode individuals co-exist in only one forest square meter (Cowling 1994; Foissner 1999; Yeates 2007; Yeates 2003). Still, they remain poorly studied in comparison to other soil taxa (Coleman and Wall 2015; Couteaux and Darbyshire 1998). Only a fraction of protist and nematode species have been described yet, and their biology and ecology remain poorly known (Brussaard et al. 2007). It thus becomes urgent to suitably recognize the species and to quantify their ecological importance for the functioning of ecosystems.
Here, as a complement to detailed reviews published in the past (Bonkowski 2004; Bonkowski and Clarholm 2012; Bonkowski et al. 2009; Chen et al. 2007), we aimed at providing a quantitative synthesis of the ecological importance of bacterivores in soil on ecosystem functions using meta-analysis tools. We intended to produce an overview of the ecological factors that are expected to drive the magnitude of their effects on ecosystem functions. Lastly, we propose a new theoretical framework based on ecological stoichiometry stressing the role of C:N:P ratios in soil, microbial and plant biomass as important parameters driving bacterivore-effects on soil N and P availability for plants, immobilization of N and P in the bacterial biomass, and plant responses in nutrition and growth.
Meta-analysis - data sources, extraction and analysis
We investigated the literature published in peer-reviewed journals before December 2014 through computer searches in Google Scholar and ISI Web of Knowledge with no restriction on the year of publication. We used the following key-words (alone or in combination): soil, biodiversity, bacteria, bacterivore, bacterial-feeders/grazers, protozoa, protists, nematodes, predators, mycorrhiza, earthworms, nodules, nutrient, nitrogen, phosphorus, microbial loop. We also searched studies in the reference lists of relevant articles and reviews. We selected studies that (i) tested the effects of bacterivores (protists or nematodes) on ecosystem functions by successfully manipulating soil bacterivores under controlled experimental conditions, (ii) measured at least one function of interest, and (iii) reported means of treatment and control. In other words, we selected studies that carefully manipulated soil bacterivores to detect causal relationships between these organisms and ecosystem functions. We thus excluded field studies that monitored soil bacterivore population changes after perturbation or during seasonal cycles, and estimated the role of bacterivores using correlative analyses. These studies could not be included for calculating an effect size. For the same reasons, we also excluded studies that eliminated soil bacterivores by adding biocides in the soil, freezing or sieving the soil without measuring the success of the elimination, studies comparing low versus high soil bacterivore densities without significant difference and modelling studies.
We analyzed the effects of bacterivores on 18 microbial and plant functions: soil microbial biomass (measured by fumigation or substrate-induced respiration), soil bacterial number (measured by plate counting), soil microbial basal respiration, microbial metabolic quotient (microbial respiration divided by microbial biomass carbon), soil phosphatase activity, soil net N mineralization, soil net P mineralization, plant growth (shoot and root biomass, shoot:root ratio) and plant nutrition (total N and P amounts expressed in mg-N or mg-P plant−1 and concentrations in shoot and root expressed in mg-N or mg-P g−1). For each study, we recorded the mean values of these functions in presence/absence of bacterivores as well as experimental information (soil parameters, species, protocols, etc.). These data were extracted directly from tables, the text or figures using PlotDigitizer 2.6.4 software.
For each observation, we calculated the individual effect size (ESi) from the natural log of the response ratio using Eq. 1 (Hedges et al. 1999).
where “T” was the treatment mean (presence of bacterivores) and “C” the control mean (absence of bacterivores) for the observation i.
This metric reflects a relative change in a function due to the presence of bacterivores. Positive and negative ESi indicated respectively a positive and a negative effect of bacterivores on the function of interest. We then calculated the mean effect size (ESm) using the Eq. 2 and estimated the 95 % confidence interval around the ESm using bootstrapping (999 iterations).
with “n” the total number of observations
ESm was considered significant (P < 0.05) if its 95 % confidence intervals did not overlap with zero. For a more intuitive understanding of the effects of bacterivores on functions, we also calculated the percentage of change from control (%CC) from the ESm. We also tested the effect of the medium used by the experimenters in their studies. To do so, we split the database into 4 groups according to the medium: “agar” (or agarose), “humus”, “sand” (mixed with organic matter or with nutrient solution) and “soil” (alone or amended with sand, organic matter and/or nutrient solution). We then calculated ESm for each group and tested for significance among groups using the Kruskal-Wallis test at the P < 0.05 level.
We also determined whether the presence of mycorrhizal fungi altered bacterivore-effects on plant nutrition and growth. We collected data from studies comparing the effects of bacterivores in the rhizosphere of plants infected or not by mycorrhizal fungi (7 studies, 14 observations). We did not include studies where the control (non mycorrhizal plant) was lacking. For each observation, we calculated the ESi for mycorrhizal and non-mycorrhizal plants and we tested for significant difference in ESm using the Kruskal-Wallis test at the P < 0.05 level.
Linear meta-regressions were performed to test the relationships between the bacterivore-induced effect size on total N (or P) amount in shoot (or root) (responses variables) and the bacterivore-induced effect sizes on shoot (or root) N (or P) concentration and shoot (root) biomass (explanatory variables). 95 % confidence intervals were calculated for each regression. The normal distribution of residuals was tested using Shapiro test. Finally, we searched for publication bias using funnel plots. We tested the significance (P-value < 0.05) of regression between the bacterivore-induced ES versus the inverse sample size of the study for each function (Peters et al. 2006). A significant regression indicated that the funnel plot was asymmetric, corresponding to possible publication bias, which was the case only for respiration (P-value: 0.02). We also tested the relationships between experiment duration, which ranged from 3 to 561 days, and ESm values using regression. Except for the concentration of N in roots, we did not find any effect of experiment duration on ESm.
Quantitative synthesis of soil bacterial-bacterivore literature
We collected 41 experimental studies published since 1977 investigating the effects of soil bacterivores on ecosystem functions (Appendix). Among them, 38 and 17 studies investigated effects of bacterivores on N and P concentrations (either in soil or plant tissues), corresponding to ~93 and ~41 % of reported studies, respectively (Fig. 1). Around 66 % of experimental studies co-inoculated bacteria and bacterivores in the rhizosphere of plants, using either herbaceous (73 %) or woody (27 %) species. According to our literature survey, in a period of 37 years, only 1.1 papers per year on average focused on the ecological functions accomplished by these organisms (without taking into account reviews and books).
This apparent lack of interest in soil bacterivores can be explained by the difficulties involved in observing, characterizing (extraction, enumeration and description) and manipulating these small-sized organisms in comparison to larger soil fauna (e.g. arthropods or earthworms) (Cowling 1994; Ekelund and Rønn 1994; Griffiths and Ritz 1988). This is particularly true for protists because the majority of taxa cannot be extracted from soils, and our taxonomic knowledge is mostly restricted to few cultivable species (Ekelund and Rønn 1994; Foissner 1999). Furthermore, this practical difficulty is enhanced by the heterogeneous and opaque nature of soils at the micro-spatial scale in contrast to aquatic environments. Soil protozoology and nematology thus require specific taxonomic skills and time-consuming microscopy in addition to expensive equipment (high-quality light-, or electron-microscopy). These methodological drawbacks together with the difficulties working under strictly sterile conditions have certainly constituted the main obstacles in the establishment of soil micro-food web experiments.
We also noted that contrasting experimental protocols have been used in these studies. Experimenters used various types of medium (agar, sand, humus or soil) or experimental devices (Petri dishes, glass tubes, rhizoboxes or pots) for the growth of organisms (Table 1). The amount and composition of organic amendments and nutrient solutions were also highly different among studies as well as the choice of model species and the duration of the experiment (from 2 to 561 days). This high diversity in protocols made the identification of factors that are likely to drive bacterivore-effects more difficult. On the other hand, it also allowed us to estimate an overall bacterivore-effect representative of a wide range of environmental conditions.
Bacterivore-effects on the soil microbial community and nutrient availability
Microbial biomass
The presence of bacterivores usually induced a reduction in soil microbial biomass or bacterial abundance (Anderson et al. 1978; Coleman et al. 1977; Darbyshire et al. 1994; Elliott et al. 1979; Zwart and Darbyshire 1992). However, a number of studies showed that the presence of bacterivores could lead to higher bacterial abundance (and biomass) due to a strong reduction of senescent cells (Elliott et al. 1980; Ingham et al. 1985; Sundin et al. 1990). The global effect of grazing on the soil microbial biomass and bacterial abundance estimated by the meta-analysis was −16 and −17 % of control, respectively (Fig. 2).
Mean effect sizes (ESm) of bacterivores on ecosystem functions. White and black circles indicate significant (different from zero) and non-significant ESm, respectively. Horizontal bars correspond to 95 % confidence intervals. Bold numbers above circles specify the ESm expressed as % of control (%CC). Italic numbers between brackets specify the total number of observations for each function. Mmic : microbial biomass; Bnum : bacterial number; Qmic : microbial metabolic quotient; Nmin : net N mineralization; Pmin : net P mineralization; S:R : shoot:root ratio
The effect of bacterivores on bacterial abundance has been shown to vary according to bacterivore species (Ingham et al. 1985; Postma-Blaauw et al. 2005), soil texture (Elliott et al. 1980), incubation time (Elliott et al. 1980), supply and availability of labile carbon (Anderson et al. 1978; Elliott et al. 1980) or nitrogen (Baath et al. 1981) and identity of bacterial taxa (Glücksman et al. 2010; Griffiths et al. 1999; Rønn et al. 2002; Xiao et al. 2010), showing that differences in bacterivore-effects are multifactorial. Certainly the long co-evolution between bacteria and protists has a major influence on the evolution of prey-capture mechanisms (Parry 2004) and bacterial defense strategies (Jousset 2011; Matz and Kjelleberg 2005).
Microbial composition
The whole composition of the soil microbial community drastically changed in the presence of protists (Bonkowski et al. 2011; Ekelund et al. 2009; Griffiths et al. 1999; Koller et al. 2013c; Rønn et al. 2002; Rosenberg et al. 2009) and bacterial-feeding nematodes (Blanc et al. 2006; Djigal et al. 2010; Djigal et al. 2004; Postma-Blaauw et al. 2005). Most published studies have a coarse taxonomic resolution limiting our ability to assess accurately grazing effects on microbial composition. Usually, the presence of bacterivores changed the relative abundance of dominant bacterial populations (Djigal et al. 2004), often increasing the proportions of bacteria that are grazing-protected, either by physical means, such as gram-positive bacteria (Griffiths et al. 1999; Rønn et al. 2002; Weekers et al. 1993) and those that make filaments such as actinomycetes (Rosenberg et al. 2009), or by chemical means (Jousset et al. 2009; Jousset et al. 2010; Jousset et al. 2008; Mazzola et al. 2009). The ability of bacterivores to alter the composition of the microbial biomass can feed back on microbial function, such as nitrification (Djigal et al. 2010; Jousset et al. 2006) or phosphatase activity (Djigal et al. 2004; Gould et al. 1979). For instance, using a DGGE technique based on PCR amplification of the amoA gene, Xiao et al. (2010) found a significant shift in the community composition of ammonia-oxidizing bacteria (AOB) from Nitrosospira sp. to Nitrosomonas sp. in presence of bacterivorous nematodes.
Selective grazing of cells has been proposed as the main mechanism causing a shift in microbial composition in the presence of protists (Bonkowski et al. 2009). Bacteria evolved different means to become grazing-resistant, which can be grouped in physical protections such as bacterial shape and size (Bjornlund et al. 2012), cell wall resistance, and the ability of cells to form micro-colonies, filaments or biofilms (Bonkowski et al. 2009; Jousset 2011); and chemical protections, such as the production of pigments (Weekers et al. 1993) or of specific toxins (Jousset et al. 2006; Jousset et al. 2009; Mazzola et al. 2009). In particular, the latter have been identified as main drivers of changes in bacterial community composition, since bacterivores have been shown to consume preferentially the competitors of the grazing-resistant taxa (Jousset et al. 2008). Protists have different sensitivities towards secondary metabolites produced by bacteria (Jousset et al. 2006). For instance, Jousset et al. (2006) found that the growth of Vahlkampfia sp. (amoeba), Neobodo designis (flagellate) and Colpoda steinii (Ciliate) on Pseudomonas fluorescens CHA0 and different exoproduct-deficient mutants was not similar among protists. The differences in growth rates of bacterivorous protist (Ekelund 1996; Weekers et al. 1993) and nematode populations (Anderson and Coleman 1981; Blanc et al. 2006; Venette and Ferris 1998) according to bacterial species suggest also that the digestibility and nutritional values of preys constitute an important trait involved in bacteria-bacterivore interactions (Pussard et al. 1994).
On the other hand, bacterivores have developed different forms of feeding (direct interception, grasping, filter-, diffusion- or raptorial feeding); this appears as an important trait involved in selective grazing aptitude (Parry 2004; Rønn et al. 2012; Weisse 2002). While ciliates move out of toxic biofilms, amoebae graze directly within biofilms and grasping and raptorial feeders rather rely on bacteria they can dislocate from biofilm surfaces, while diffusion and filter feeders rely on the free-swimming bacteria (Rønn et al. 2012). In addition, certain bacterivore nematode taxa evolved a grinder in the terminal bulb which serves for crushing trapped bacteria (Fürst von Lieven 2003), thus providing a physical means to consume bacteria with thick cell walls that are largely undigestible for other bacterivores, like gram-positive bacteria.
Because bacterial-feeding nematodes can ingest around 20 cells at each suction (Ferris et al. 1997), it appears unlikely that they actively select for specific prey taxa as protists do. Jousset et al. (2009), using mixed populations of mildly toxic wild-type and non-toxic gacS-deficient mutants of Pseudomonas fluorescens CHA0 in batch and rhizosphere systems, showed that the diet composition of the nematode Caenorhabditis elegans did not vary with changes in the frequency of the two bacterial strains, confirming its lack of selective ability compared to Acanthamoeba castellanii that clearly preferred the non-toxic bacterial mutants. Accordingly, bacterial-feeding nematodes are more likely to alter bacterial community composition through passive mechanisms. The high variations in the head shape of Cephalobidae species recorded by De Ley (1992) supports this hypothesis. He investigated in detail how the shape of the labial probolae and the development of cephalic probolae at the nematode head allow passive food specialization, thereby optimizing niche partitioning among co-occurring bacterivore nematode species. According to the shape of their probolae, nematodes species may thus change the size of different bacterial populations, and may alter biological interactions among bacteria, leading to bacterial community composition shifts. Lastly, bacterivorous nematodes have been suggested to modify the amount and composition of rhizodeposits, especially carbohydrates which increased 2.6 times in presence of nematodes, and to increase the allocation of net carbon production in rhizodeposition (Sundin et al. 1990). This can in turn feed back on the composition of the bacterial community (Benizri et al. 2002; Puglisi et al. 2013).
Microbial activity and nutrient availability
The presence of bacterivores generally enhances the overall microbial activity and turnover (Alphei et al. 1996; Coleman et al. 1978; Djigal et al. 2004; Kuikman et al. 1990). The meta-analysis revealed that the presence of bacterivores significantly increased soil microbial basal respiration and microbial turnover, measured as microbial metabolic quotient (respired-carbon relative to microbial biomass carbon) by +29 and 35 % of control, respectively (Fig. 2). It is thus possible that the microbial carbon-use efficiency decreases in presence of bacterivores (Manzoni et al. 2012; Sinsabaugh et al. 2013). The main mechanisms are that (i) bacterivores can ingest senescent bacteria and contribute to maintain younger bacteria cells with higher metabolic activity (see discussion in Bonkowski (2004)) and (ii) they release undigested food particles, labile carbon and nutrients making the medium more favorable for bacterial re-growth (Griffiths 1994; Pussard et al. 1994).
The effects of bacterivores on microbial communities have strong consequences for soil nutrient availability (Clarholm 1985a; Koller et al. 2013b; Kuikman and Van Veen 1989; Xiao et al. 2010). We found that the presence of bacterivores almost doubled soil N (Nmin) mineralization (×1.8 in absence of plants) (Fig. 2). Two main pathways of bacterivore-effects on soil nutrient availability do exist simultaneously: excretion of nutrients (direct way) according to the consumer-driven nutrient recycling theory (Elser and Urabe 1999) and stimulation of the microbial activity and turnover as quoted earlier (indirect way).
Using stable isotopes, Crotty et al. (2013) found that soil protists only have a production efficiency of 37 %. Ferris et al. (1997) found higher production efficiencies (ranging from 58 % to 86 %) for eight bacterivore nematodes species. Because bacterivores have to maintain stoichiometric homeostasis relative to their food, and loose carbon due to respiration, a great part of the ingested N will be ultimately released by bacterivores (Anderson et al. 1983; Darbyshire et al. 1994; Ferris et al. 1997; Ferris et al. 1998). Borkott (1989) found mass C:N ratios of 3.5:1, 4.7:1 and 5.6:1 for Naegleria gruberi (amoeba), Colpoda steinii (ciliate) and Rhabditis sp. (bacterivore nematode), respectively. Using recent values of global soil microbial stoichiometry (mass C:N ratios ranging from 5.5:1 to 7.4:1) (Cleveland and Liptzin 2007; Xu et al. 2013) and production efficiencies from Crotty et al. (2013) and Ferris et al. (1997), we calculated, using the same procedure as Griffiths (1994) and Ferris et al. (1997), that theoretical ranges of N excretion would be 23–32 %, 43–49 % and 9–20 % of assimilated-N for amoebae, ciliates and nematodes, respectively. The C:N ratios of both bacteria and bacterivores will thus directly influence the amount of assimilated-N excreted by the bacterivores (Elser and Urabe 1999).
Furthermore, the presence of bacterivores will increase microbial respiration and turnover (Coleman et al. 1978; Levrat et al. 1992), leading to lower microbial C:N ratio. In addition, the C:N ratio of bacteria determines the respiration rates in presence of bacterivores, with C-losses increasing at decreasing C:N ratios (De Telegdy‐Kovats 1932) leading to higher N release by bacterivores at narrow C:N ratios of the prey. However, the soil microbial biomass C:N:P has been shown to be relatively stable compared to its resource (Cleveland and Liptzin 2007; Xu et al. 2013); and microbes keep strict homeostasis in respect to nutrient availability in soils (Griffiths et al. 2012). To maintain their stoichiometry, microbes can regulate their nitrogen-use efficiencies by releasing elements in excess depending on their carbon-use efficiency (Mooshammer et al. 2014a). The excess N may then become available for roots in presence of bacterivores (Kuzyakov and Xu 2013).
However, small changes in microbial biomass stoichiometry can occur after shifts in the microbial community structure (Fanin et al. 2013) and over-saturation of microorganisms with P has been observed in soils with high P levels (Griffiths et al. 2012). Fast-growing bacteria (copiotrophs) have been suggested to exhibit lower biomass C:N:P ratios (higher nutrient requirements) than slow-growing ones (oligotrophs) (Elser et al. 2003; Hodge et al. 2000). In line of the growth rate hypothesis (Elser et al. 2003), N:P ratios in organism decrease with increasing growth rates due to elevated demands for P for the synthesis of P-rich ribosomes. Therefore, shifts in the dominance of fast- or slow-growing bacteria induced in presence of bacterivores can transfer to shifts in microbial biomass stoichiometry, with important consequences on the content of N and P available for plant growth. It is important to note that the strict homeostatic behavior of the microbial biomass C:P ratio has been observed with the concentration of available P in soil rather than with total soil P (Griffiths et al. 2012). The experimenter’s ability to quantify, using different chemical extractors, the size of C, N and P pools available for bacteria is thus crucial for the evaluation of soil bacterial biomass stoichiometric behavior.
Ferris et al. (1998) calculated that bacterial-feeding nematodes excreted NH4-N with rates ranging between 0.0012 and 0.0058 μg-N ind.−1 day−1 according to species, but in addition, considerable amounts of organic N can be released by nematodes (Anderson et al. 1983). Wright (1975) found that that 3–14 %, 15–34 % and 35–45 % of the total N (mean 13.7 μmoles N g−1 nematode fresh wt h−1) liberated in Ringer solution by the nematode Panagrelus redivivus were composed of urea, amino acids and proteins, respectively, while ammonium contributed 23-35 % of total N-release. Large releases of organic N (in total and in proportion of total N) by nematodes may occur when nematodes exhibit high nitrogen-use efficiency in order to balance their stoichiometry to bacterial biomass C:N:P ratios, explaining why Anderson et al. (1983) observed declines in excretion of organic N with decreasing resource availability (i.e. when nematode populations became older). Knowing that dissolved organic N (especially low molecular weight N compounds) can be re-used by soil bacteria or taken up by plants (Gallet-Budynek et al. 2009; Nasholm et al. 2009; Neff et al. 2003), bacterivore-effects on soil N availability likely have been under-estimated because experimenters generally focused only on NH4.
Interestingly, we found a great variability in ESi for Nmin as shown by the 95 % confidence interval. In parallel, we found that when the substrate used in experiments was soil, the ESm was very high in comparison to humus, while in sand it exhibited intermediate values (Fig. 3a). Humus had the highest mass C:N ratios (mean ~39:1) while soils had the lowest ones (mean ~15:1) and sand exhibited intermediate values (mean ~20:1). We therefore suggest that bacterial growth in substrates with high C:N ratios (humus), and in absence of living roots, will be mostly limited by N, leading to rapid immobilization of N released by bacterivores. In contrast, in substrate with low C:N ratios (soil), bacterial growth will be limited by C and bacterial N immobilization should decrease (Ferris et al. 1998).
We also found that protists exhibited significant higher effects than nematodes on Nmin but the highest values were found when both bacterivore groups were present in the microcosms (Fig. 4a). Accordingly, the choice in bacterivore species appears as an important experimental parameter explaining the magnitude of bacterivore-effects on Nmin. The broad classification of bacterivores is by far not accurate enough to predict how bacterivores impact soil N cycling. For instance, Ferris et al. (1998) found high variation of different nematodes species to net N mineralization. Predicting bacterivore-effects on nutrient availability therefore remains difficult, especially when a mixture of bacterivore species is used.
Changes in bacterivore-induced effect size (mean) on shoot N mineralization (a), root biomass (b) and shoot N concentration (c) according to the presence of bacterial-feeding protists (Pr), nematodes (Nem) alone or together (Pr + Nem). Letters (a and b) indicate significant difference in ESm among media types according to a Kruskal-Wallis test at P < 0.05
Applying the same stoichiometric reasoning as for N (Borkott 1989; Cleveland and Liptzin 2007; Crotty et al. 2013; Ferris et al. 1997; Xu et al. 2013), theoretical ranges of excess-P excretion would be 27–48 %, 73–81 % and 66–76 % of assimilated-P for amoebae, ciliates and nematodes, respectively. These estimates are higher than for N (except for nematodes) while, in the meta-analysis, we found lower bacterivore-effects on P mineralization (Pmin) (22 % of control) in comparison to N (Fig. 2).
The theoretical liberations of N and P by bacterivores did not take into account biotic (microbial immobilization, microbial carbon-use efficiency, resource stoichiometry, etc.) and abiotic processes (adsorption of P, leaching of N, etc.) occurring after nutrient excretion by bacterivores, thus differentiating gross from net effects of bacterivores on nutrient availability. In humus (where the C:P ratio is high), the bacterivore-effect on Pmin was negative, while in soil (where the C:P ratio is low) we found a positive effect of bacterivores on Pmin (Fig. 3b). Hence, in a high C:P ratio environment, bacterial growth may be mostly limited by P and rapid immobilization of excreted P from bacterivores may have occurred. However, in contrast to N, P is poorly mobile in the soil solution and can be rapidly adsorbed by soil colloids (Hinsinger et al. 2011), reinforcing rapid immobilization of P in the microbial biomass and hampering the overall positive net effect of bacterivores on Pmin.
Bacterivore-effects on plant performance
Plant growth and root architecture
Positive effect of bacterivores on plant growth have been observed for both herbaceous (Alphei et al. 1996; Djigal et al. 2004; Krome et al. 2009a) and woody (Bonkowski et al. 2001b; Irshad et al. 2011; Jentschke et al. 1995) plant species (Table 1). Our meta-analysis revealed a significant effect of bacterivores on shoot (+27 % of control) and root biomass (+21 % of control) without affecting the shoot:root ratio (Fig. 2). Interestingly, the 95 % confidence interval did not exhibit huge variation though many ecological factors have been suggested in the literature to affect bacterivore-effects on plant growth. Both, protists and nematodes induced root growth of similar magnitude, but when both were present, their effects were significantly larger (Fig. 4c). We did not find such an additive effect for shoot biomass (Fig. 4b; P-value = 0.109).
Besides biomass, the presence of protists in the rhizosphere of plants has been shown to alter profoundly root architecture by promoting lateral root production (Jentschke et al. 1995; Kreuzer et al. 2006) (Table 1). Based on 11 studies, we found that bacterivores increased the number of root tips by 96 % of control. However, this positive effect was greatly variable among studies (Table 1). Shifts in root architecture were also observed for bacterial-feeding nematodes but to a lesser extent than for protists (Cheng et al. 2011; Irshad et al. 2012; Irshad et al. 2011). For instance, while protists increased the number of root tips on average by a factor of 2.5, nematodes only increased the number of tips by a factor of 1.2 (Table 1). The presence of mycorrhizal fungi usually reduced the bacterivore effect on root length, specific root length, root area and number of root tips.
Plant nutrition
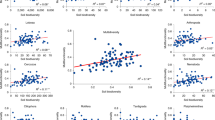
We found substantial bacterivore-effects on the total amount of N in shoots and roots, i.e. +5 and +28 % of control (Fig. 2). Although the increase of plant N concentration in shoots by bacterivores was large (+25 % of control) it was highly variable and not different from 0 (non-significant effect on this variable). By conducting linear meta-regressions, we found that ~34 % (P < 0.001) and ~65 % (P < 0.001) of the total variance of ESm on total N amount in shoot (response variable) was explained by bacterivore-effect on shoot biomass and shoot N concentration (explanatory variables), respectively, without significant interaction between the two explanatory variables (Fig. 5a). These results imply that in some experimental conditions, bacterivores enhanced the total shoot N amount by increasing the shoot N concentration while in others, bacterivores increased the shoot N amount by increasing shoot biomass, explaining why we found high variance in ESm for shoot N concentration. Exceptions are the studies by Kuikman et al. (1991) and Alphei et al. (1996) where increased plant biomass production in presence of protists or nematodes was neither paralleled by an increased total uptake of plant N, nor by increased concentrations of N.
Multiple linear regressions performed on individual effect-size (ESi). a ESi of total N amount in shoot (response variable) as a function of ESi of shoot biomass and ESi of shoot N concentration (explanatory variables). b ESi on total P amount in root (response variable) as a function of ESi on root biomass and ESi on root P concentration (explanatory variables). %expl. indicates the percentage of total variance of the response variable explained by each explanatory variable and by their interaction. ***P < 0.001 (n = number of observations)
Interestingly, we found higher shoot N concentrations in experiments with bacterial-feeding nematodes than with protists (Fig. 4c). These results suggested that the increase in total shoot N amount was mostly caused by higher N concentrations in plant tissue when nematodes were inoculated. Together with the lower effects of nematodes on root architecture, the data suggest different mechanisms of plant responses to bacterivore nematodes or protists, and might explain why the combined effects of both bacterivore groups were generally larger than the effects of either group alone.
In respect to P, we found that bacterivores increased the total amount of P in shoots and roots by 38 and 55 % of control, respectively (Fig. 2). The data suggest differences in the internal plant cycling of newly-acquired N and P: the bacterivore-induced gain of N in plant tissues was mostly allocated to shoots (probably for the maintenance of high carbon fixation rates via photosynthesis) while the gain of P was more allocated to roots. Krome et al. (2009a) and Koller et al. (2013c) showed that the effects of protozoa on plant performance were more related to enhanced plant carbon fixation rather than nitrogen uptake. Compared to N, the effects of bacterivores on shoot P concentration (+30 % of control) were less variable and different from 0, suggesting that surplus P made available by bacterivores was commonly concentrated in plant tissues. Although the presence of bacterivores led to an important increase in root P concentration (+23 % of control), this effect was highly variable. The meta-regression showed that ~16 % (P < 0.001) and ~75 % (P < 0.001) of the total variance of ESm on total P amount in roots (response variable) was explained by bacterivore-effects on root biomass and root P concentration (explanatory variables), respectively without significant interaction between these two explanatory variables (Fig. 5b). Some experimental conditions, which remain to be known, may thus favor bacterivore-effects on nutrient concentration in plant tissues (without affecting plant biomass) and others may promote bacterivore-effects on plant biomass (and increase total N and P amount in plants).
Plant reproduction & defense
It is not surprising that positive effects of bacterivores on nutrient uptake and biomass of plants will lead to increased plant reproduction. For instance, the presence of protozoa increased the number of ears (+60 %), seeds (24 %) and the individual seed weight (+32 %) of barley plants, and the crop was even more tolerant to aphid herbivores (Bonkowski et al. 2001a). More recently, Krome et al. (2009a) showed that A. castellanii enhanced the seed production of Arabidopsis thaliana (Brassicaceae) more than 3- and 7-fold compared to treatments with bacteria and sterile grown plants, respectively. Unfortunately, only these two papers investigated bacterivores effects on plant reproduction.
Relationships between bacterivores and root-infecting symbionts
Bacterivores & mycorrhizal fungi
The effects of bacterivores on plant functions have been mostly investigated in the rhizosphere of non-mycorrhizal plants (84 % of reported studies) (Fig. 1). This is a severe shortcoming as more than 80 % of herbaceous and woody plants are infected by soil fungi, mostly forming arbuscular mycorrhizal (Smith and Read 2008) and ectomycorrhizal symbioses (Marmeisse et al. 2004).
The formation of mycorrhizal roots is considered as the most widespread response of plants to low soil nutrient availability (Plassard et al. 2011). By prospecting a large volume of soil away from plant roots, and by releasing carboxylates (Finlay 2008) and enzymes (Courty et al. 2006; Louche et al. 2010), mycorrhizal fungi have been often shown to significantly enhance plant N and P acquisition (Plassard and Dell 2010; Tibbett and Sanders 2002; Wallenda and Read 1999).
The presence of mycorrhizal fungi can alter the positive effects of bacterivores on plant performance through two main pathways. First, the growth of bacteria and bacterivores clearly depends on plant carbon allocation to roots. It has been estimated that ca. 7-30 % of net carbon fixation will be directly allocated to the root-infecting fungal symbionts by the host plant (Jones et al. 2009; Leake et al. 2004). It is likely that the amount of carbon released by root exudation will decrease in the presence of a mycorrhizal partner (Meier et al. 2013; Olsson et al. 1996) with indirect detrimental effects on bacterivore populations. Secondly, mycorrhizal fungi are known to profoundly change root biomass and architecture (Brown et al. 2013). Usually, the presence of the mycorrhizal symbiont reduced the stimulating effect of bacterivores on lateral root production (Table 1). Bacterivore effects on plant nutrition through more complex root architecture can thus be altered by mycorrhizal fungi.
However, this a priori hypothesis could not be confirmed by the meta-analysis. We did not find any negative effect of mycorrhizal fungi on bacterivore effects on N and P concentrations or total amount in plant tissues. In contrast, the ESm of bacterivores on shoot and root biomass were significantly reduced when the plants were in association with mycorrhizal fungi (Fig. 6). This negative effect of mycorrhizal fungi on ESm was very low for shoot biomass (−5 %) but substantial for root biomass (−104 %), showing that even when mycorrhizal fungi reduced the positive effect of bacterivores on root growth, the positive effects of bacterivores on plant nutrition were still maintained. The few existing studies support this hypothesis for both plants infected by arbuscular (Koller et al. 2013b; Koller et al. 2013c) and ectomycorrhizal fungi (Bonkowski et al. 2001b; Irshad et al. 2012). These results also support the hypothesis formulated by Bonkowski et al. (2001b) assuming that the mycorrhizal and the bacteria-bacterivore mutualisms complement each other and plant resources are allocated to optimize simultaneous exploitation of both mutualistic relationships. Because studies are lacking, it was not possible to distinguish the effect of each mycorrhizal fungi type (arbuscular-, or ecto- mycorrhiza) on bacterivore-effect sizes. Knowing that both fungal symbionts are phylogenetically and physiologically distinct, this constitutes a serious shortcoming. More studies are required, especially those focusing on different types of mycorrhizal fungi in interaction with bacterivores.
It is well known that the ability of plants to take up organic N as source of N is greater in presence of mycorrhizal fungi (Cappellazzo et al. 2008; Nasholm et al. 2009; Neff et al. 2003; Plassard et al. 2000; Wallenda and Read 1999). Because significant amounts of dissolved organic N (amino acids) are released by bacterial-feeding nematodes (Anderson et al. 1983; Sundin et al. 1990; Wright 1975), we expect that the presence of mycorrhizal fungi will favor the acquisition of some organic N- (and maybe P-) forms released by bacterivores, before nutrients can be re-used by rhizosphere bacteria according to their biomass C:N:P ratios. We did not find experimental study testing this hypothesis, but we believe that this organic N circuit should not be overlooked, and may constitute an important mechanism by which bacterivores improve mycorrhizal plant N nutrition.
Bacterivores & N2-fixing plants
Because N usually limits plant productivity, biological N fixation is one of the most important functions of terrestrial ecosystems. Yet, little attention has been given to the potential role of bacterivores on symbiotic N fixation and legume growth (Appendix). Ramirez and Alexander (1980) showed that protists decreased the abundance of root-nodule bacteria in the rhizosphere of Phaseolus vulgaris. Lennox and Alexander (1981) found higher numbers of root nodules and growth of P. vulgaris when they used a biocide to suppress protists, but we can not judge the side-effects of the biocide, since no protist-specific biocides exist. More recently, Horiuchi et al. (2005) showed that the legume Medicago truncatula, by producing volatile dimethylsulfide, attracted bacterivorous nematodes (Caenorhabditis elegans) which significantly increased the transfer of Sinorhizobium meliloti to the roots, and subsequently, the initiation of the N-fixing symbiosis in legumes. Soil bacterivores, especially nematodes, can thus act as vectors of rhizosphere bacteria and increasing root colonization by rhizobia. Also, high symbiotic N fixation efficiency usually requires high levels of available P because high rates of energy transfer take place in the symbiotic nodule (Drevon and Hartwig 1997; Vitousek et al. 2002). The meta-analysis showed that bacterial bacterivores increased soil P availability on average by 22 % of control. We can hypothesize that protists and nematodes, by increasing soil P availability and subsequent plant P acquisition, might promote higher N fixation efficiency once the nodules are formed. The N:P ratio of nodules would then be an important proxy to predict how bacterivores in the vicinity of nodules may affect plant N uptake.
A ‘stoichiometric’ perspective on bacteria-bacterivore-plant interactions
Stoichiometric controls on bacterivore-effects on soil nutrient availability
Comparing the data of bacterivore effects on N- and P-availability points to an important role of stoichiometry if we want to predict how bacterivores impact ecosystem functions.
(1) Bacterivores have to maintain stoichiometric homeostasis, but especially protists have been shown to exhibit low production efficiency (Crotty et al. 2013). Accordingly bacterivores release a great part of ingested N and P in mineral form (Elser and Urabe 1999). The amounts of N- and P-release are expected to vary according to bacteria and bacterivores C:N:P ratios. For example, Cole et al. (1978) showed that amoebae were highly efficient at recycling microbially immobilized P to soil in comparison to bacterivorous nematodes.
(2) Bacterivores often increase microbial turnover. Microbes are homeostatic in terms of their biomass C:N:P at the community scale (Cleveland and Liptzin 2007) and can regulate their nutrient-use efficiency by releasing elements in excess depending on their carbon-use efficiency (Mooshammer et al. 2014a) leading to a strict homeostasis with the available N- and P-levels in soil (Griffiths et al. 2012).
(3) Bacterivores can alter the soil microbial community structure. According to the growth rate hypothesis (Elser et al. 2003), C:N:P ratios in organism vary with growth rates. Therefore, small changes in microbial stoichiometry can occur after shifts in the microbial community structure (Fanin et al. 2013) caused by bacterivores.
(4) Soils with high C:N or C:P ratios may be nutrient deficient for bacterial growth, favoring rapid immobilization of newly-mineralized N or P from bacterivores (Ferris et al. 1998). Soils with low C:N or C:P ratios may induce energetic starvation for bacterial growth, reducing bacterial N and P immobilization. The net effects of bacterivores on soil N and P mineralization therefore depend on energy available in terms of labile plant C, either provided by plant roots, or derived during initial phases of litter decomposition (Bonkowski and Clarholm 2012).
Stoichiometric controls on bacterivore-effects on plant nutrition and growth
Clarholm (1985b) and Bonkowski (2004) proposed theoretical frameworks describing how bacterivores impact plant functions. The ‘soil microbial loop’ hypothesis (Clarholm 1985a) described nutritional mechanisms, quoted earlier, by which bacterivores may increase plant nutrition and growth. Because bacterivores can enhance root growth without increasing plant nutrient concentrations (Alphei et al. 1996; Jentschke et al. 1995; Kuikman et al. 1991), Bonkowski (2004) proposed a ‘hormonal’ hypothesis based on non-nutritional mechanisms where protists increase lateral root branching by altering microbe-root communication, with strong consequences on plant internal auxin metabolism (Krome et al. 2009b). Both nutritional and non-nutritional mechanisms will occur concurrently in the rhizosphere of plants and are not exclusive of each other. However, it is unlikely that they contribute to the same magnitude to bacterivore-effects under different experimental conditions. For instance, we showed that the increase in total N amount in shoots induced by bacterivores was either explained by higher shoot N concentration or higher shoot biomass. We found similar partitioning in explanatory variables for bacterivore-effects on total P amount in roots. We believe that ecological stoichiometry can help to understand these patterns.
Ecological stoichiometry describes the importance of the relative proportions between elements for the growth of organisms (Elser et al. 2000; Gusewell 2004; Sardans et al. 2012; Zechmeister-Boltenstern et al. 2015). Plant C:nutrient ratios are assumed to decrease with increasing plant relative growth rate (Ågren 2004; Elser et al. 2000). This was conceptually presented by Ågren (2008) as a linear relationship between these two plant traits (Fig. 7a). From this graph, we defined the “critical ratio” (Rcri) which designates the C:nutrient ratio above which plant growth is null (nutrient limitation).
Theoretical framework describing the relative dominance* of nutritional and non-nutritional mechanisms involved in bacterivore-effects on plant performance. a Relationship between plant relative growth rate and plant C:nutrient ratios (modified from Ågren (2008)). The solid line describes the C:limiting-nutrient (N or P) ratio while the broken lines indicate three different variations in the C:nutrient ratio of a second element (N or P) as a function of relative growth rate. We here defined the critical C:limiting-nutrient ratio (Rcri) above which the relative growth rate is null. b Diagram illustrating four different cases of plant behavior in the presence of bacterivores along a soil C:N and C:P gradients. Solid lines describe the C:Ncri and C:Pcri according to soil C:N:P ratio, while the broken line indicates a hypothetical zone where the dominant mechanism changes. Grey arrows describe the hypothetical trends in microbial nitrogen- and phosphorus-use efficiency (NUE, PUE) along the soil C:N:P gradient. *the term “dominance” describes the relative importance of mechanisms to initiate and drive the effects of bacterivores on plants at a given moment in the experiment
For a certain plant species, the major cause of natural high C:nutrient ratios in plant tissues is assumed to be nutrient availability in soil (Ågren 2008). Nutritional mechanisms by bacterivore activity rely on a relatively high release of nutrients from consumed microbial biomass. Such conditions are experimentally met when detritus of narrow C:nutrient ratio is added to soil, supporting high levels of microbial growth and a subsequent substantial release of nutrients by bacterivores (Bonkowski et al. 2000). When the readily available organic C from the amendments is depleted, microbial biomass turnover will release nutrients for plant uptake, and nutrient release will be further enhanced in presence of bacterivores.
Under more natural soil conditions the nutritional mechanisms should prevail when nutrients are poorly available for plants, i.e. when competition for N and P between microbes and plants is strong. For example, Koller et al. (2013a) added litter of high C:N ratio (i.e. low quality, LQ) or low C:N ratio (high quality, HQ) to soil inoculated with bacteria or with bacteria and the amoeba A. castellanii, and planted with Plantago lanceolata. By 13C-labeling of the plants, they showed that plants in presence of high C:N ratio substrates allocated 12 % more recently fixed photosynthates to roots in presence of protists compared to controls. Subsequently, 13CO2 respiration losses from the soil more than doubled, because the excess release of root C could not be fixed in microbial biomass due to nutrient deficiency. Here the presence of bacterivores caused a 17 % decrease in plant C:N ratio which was likely due to two parallel processes, an increased nitrogen uptake and an excess energy expenditure towards rhizosphere C-allocation.
The non-nutritional mechanisms suggest that bacterivores stimulate higher root production and relatively more C-fixation before or without an increase in plant nutrient concentration. The formation of lateral roots and the resulting root branching requires substantial plant investment of photosynthates and nutrients: N for proteins, P for ribosomes and energy synthesis. Manipulating nutrient availability to plants through the addition of HQ litter to soil, Koller et al. (2013a) found a substantially higher increase of root biomass (x1.8) (and increased root surface area) compared to shoot biomass (x1.3) in treatments with bacterivores compared to control. In contrast to the LQ treatment, the plant C:N ratio increased by 14 %, i.e. plant biomass increased while the nutrient concentration decreased. This relatively wider plant C:N ratio might have been a result of a more efficient photosynthesis. At the same time, due to greater nutrient availability in low C:N substrates, microbial biomass in the plant rhizosphere incorporated substantially more 13C from recent photosynthates. This may be observed when nutrients are highly available for plants, i.e. when competition for N and P between microbes and plants is low.
The relative dominance of nutritional and non-nutritional mechanisms induced by presence of bacterivores can be plotted along a soil C:N and C:P co-gradient likely to drive (i) plant-microbe competition towards N and P and (ii) plant stoichiometry status at the beginning or during co-inoculation experiments (Fig. 7b). The term dominance refers to the relative importance of mechanisms to initiate and drive bacterivore-effects on plants. Total soil carbon and nutrient concentrations are poor predictors for energy and nutrient availability to bacteria (e.g. carbon compounds can be either easily or hardly decomposable). In this model, we thus reasoned on C, N and P pools potentially available for bacteria.
We distinguished four contrasting cases. In case 1, plants exhibit C:N and C:P ratios above their respective critical ratios, and are strongly limited by N and P (the relative growth rate is therefore null). This case may happen when plants grow in soils with high C:N and C:P ratios. Under such soil conditions, microbes are strongly limited by N and P and expected to exhibit high nitrogen- and phosphorus-use efficiencies (Mooshammer et al. 2014b), and consequently the competition for N and P will be shifted in favor of microbes (Kuzyakov and Xu 2013). The experiment of Koller et al. (2013a) cited above, demonstrates that plants under nutrient limitation will allocate relatively more photosynthates belowground, thus providing energy for potential root symbionts or priming effects on soil organic matter. Under these circumstances the consumption of microbes by bacterivores will lead to increased microbial turnover and may decreased microbial carbon-use efficiency. Plants will primarily benefit from the released N and P by bacterivores. In cases 2a and 2b, plants have C:N or C:P ratios just below their respective critical ratios; they are thus strongly limited by N or P according to soil C:N:P ratios. Similar mechanisms as described above may occur for other plant limiting nutrient concentrations, but according to plant species stoichiometric behavior, an excess uptake of the non-limiting nutrient may also occur (Ågren 2008). In cases 3a and 3b, plants grow in soils with intermediate C:N or C:P ratios, respectively. Microbes become limited by carbon and their nitrogen- and phosphorus-use efficiencies may decrease, while plants are limited by N or P but their C:nutrient ratios are below the critical ratios. The presence of bacterivores, and the subsequent changes in nutrient availability and root architecture, may result in both nutrient storage and biomass production, according to species stoichiometric behavior (co-dominance of mechanisms). In case 4, microbes are strongly limited by carbon (nutrient-use efficiency is expected to be low) and plants exhibit low C:N and C:P ratios. This may happen in substrates with low C:N and C:P ratios (or soils with low availability of labile C). Plant nutrient concentrations are high enough to cause an increase of plant biomass (dilution of plant nutrient concentration can occur), but also lateral root production (i.e. root surface area) for more efficient nutrient uptake in response to bacterivores (dominance of non-nutritional mechanisms). This case is expected to happen when plants grow in a relatively nutrient-rich environment (Jentschke et al. 1995), or when plants experience nutrient imbalances which is critical, especially at early life stages (Fenner and Lee 1989; Hanley and Fenner 1997).
Conclusion & Perspectives
In this meta-analysis, we showed that bacterivores in soil contributed significantly to numerous key soil and plant functions. We suggest a theoretical framework based on ecological stoichiometry stressing the role of C:N:P ratios in soil, microbial and plant biomass as important parameters driving bacterivore-effects on soil N and P availability for plants, immobilization of N and P in the bacterial biomass, and plant responses in nutrition and growth. Unfortunately, it has not yet been possible to test this ‘stoichiometric’ hypothesis using meta-analysis tools because of insufficient data on soil and plant C:N:P ratios. More co-inoculation experiments focusing on bacterivore-effects on plant nutrient acquisition in various environmental conditions are therefore needed. In addition, we propose three outlooks to contribute to our understanding of bacterivore functions in terrestrial ecosystems.
Varying species models to deeper understand ecological roles of bacterivores –
Studies investigating the effects of bacterivores on ecosystem functions usually used one model species. In this meta-analysis, we found that 57 % of studies focusing on protists effects selected the A. castellanii species as a model, corresponding to 28 % of all studies reported in the meta-analysis. This species was frequently used because Acanthamoeba are opportunist protists with worldwide distribution (Geisen et al. 2014) that appear frequently in serial dilutions and are easy to obtain in cultures. Concerning bacterial-feeding nematodes, the genera Mesodiplogaster, Rhabditis, Acrobeloides (and several other Cephalobidae genera), were frequently used. However, the responses of soil and plant functions to protist and nematode occurrence are likely to diverge according to species used by the experimenters. Indeed, it is recognized that the effects of bacterivores on microbial community composition (Djigal et al. 2004; Rønn et al. 2002), soil N mineralization (Ferris et al. 1998) or plant performance (Bonkowski and Clarholm 2012; Cheng et al. 2011; Irshad et al. 2011) are species-specific. It is thus urgent to conduct further studies using other model species such as Cercomonads, Kinetoplastids or Chrysophytes.
From population to community-level experiments
We also found that on average 38 % of studies used a mixture of different protists or nematodes species. Only 6 studies (15 %) investigated soil protists and nematodes together and only 2 of them did it in the rhizosphere of a plant (Fig. 1), biological interactions among bacterivores are diverse and often antagonistic (Rønn et al. 2012). Recent studies indicate that intraguild-predation between ‘bacterivores’, especially antagonistic interactions between protists and nematodes are quite common and not unidirectional (Geisen et al. 2014; Geisen et al. 2015). Bacterivore nematodes are known to prey on protists (Anderson et al. 1978; Bonkowski et al. 2000; Neidig et al. 2010; Rønn et al. 2012) and vice-versa (Bjornlund and Rønn 2008; Neidig et al. 2010). The population density of one bacterivore taxon can thus increase on the cost of other another bacterivore (Anderson and Coleman 1981). Unfortunately, the effect of within-trophic group diversity of the soil bacterivore community on ecosystem function has rarely been investigated (Postma-Blaauw et al. 2005; Saleem et al. 2012). As a consequence, we are still far from predicting which bacterivore community attribute(s) drive(s) the overall effect of the bacterivore community on ecosystem functions. A number of community attributes might potentially drive the bacterivore community effects, such as (i) species richness, (ii) keystone groups with specific ecology (e.g. amoebae), (iii) keystone species (e.g. Acanthamoeba castellanii), (iv) particular trait dissimilarity (head morphology, feeding types, body size, demographic parameters; etc.) or (v) multi-trait dissimilarity (Rao’s dissimilarity coefficient). Identifying the most relevant attributes of soil bacterivore communities driving ecosystem functions should help us to get closer to predicting the actual effects of bacterial bacterivores in the fields.
Kinetic experiments to monitor bacterivore-induced effects on plant performance
Most studies investigated the effect of bacterivores on plant performance after a specific period of growth. Kinetic experiments are scarce because monitoring bacterivore-effects on plant growth or nutrition requires a high number of replicates, which is difficult from a technical point of view. However, this type of studies might provide crucial information on the sequence of plant and microbial responses in the presence of bacterivores, and the dynamics and persistence of bacterivore-effects on ecosystem functions (Krome et al. 2009a). For instance, Ingham et al. (1985) observed a temporary effect of nematodes (Pelodera sp. and Acrobeloides sp.) on plant (Bouteloua gracilis) growth. Kinetic experiments conducted under contrasting soil N and P availability would be optimal to test the stoichiometric hypothesis.
Emphasize on P and ecological factors
P is essential for plant growth and may be limiting in many environments. In contrast to N, P is characterized by its relative immobility in soil and the very low concentration of its available form in the soil solution (Hinsinger 2001; Hinsinger et al. 2011). Here, we found that N was 2.3-times more studied than P in both soil and plant tissues. This is a serious deficiency given that plant P acquisition efficiency is often based on biological interactions between plant roots and rhizosphere organisms. Using P radio-isotopes is probably the best way to better understand how bacterivores affect P cycling at the soil-plant interface. Also, the literature indicates that a large number of other ecological factors are likely to impact bacterivore-effects on ecosystem functions: bacterivore species (Cheng et al. 2011; Ferris et al. 1997), soil properties (Ekelund and Rønn 1994), plant species, or cultivars (Somasundaram et al. 2008), the presence of symbiotic mutualists (Herdler et al. 2008; Irshad et al. 2012) or other free fauna (Bonkowski and Schaefer 1997; Tao et al. 2009), time after inoculation (Krome et al. 2009a), etc. Unfortunately, the number of observations for each factor was too low or difficult to aggregate to perform a meta-analysis. For instance, it was impossible to test the effect of initial bacterivore biomass (or density), soil properties (pH, texture, carbon content, water content; etc.), plant species or cultivar, or the presence of rhizosphere organisms (e.g. mycorrhizal fungi or rhizobia) on bacterivore-induced effects on ecosystem functions. Conducting more detailed studies on bacteria/root/bacterivore interactions should deeply improve our understanding of soil food web roles in ecosystems functions, and ultimately help us to predict how the erosion of soil biodiversity will affect ecosystem services in the future.
References
Adl SM, Simpson AGB, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup Ø, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MFJR (2005) The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eucaryot Microbiol 52:399–451
Adl SM, Simpson AG, Lane CE, Lukeš J, Bass D, Bowser SS, Brown M, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, leGall L, Lynn DH, McManus H, Mitchell EAD, Mozley-Stanridge SE, Wegener Parfrey L, Pawlowski J, Rueckert S, Shadwick L, Schoch C, Smirnov A, Spiegel FW (2012) The revised classification of eukaryotes. J Eucaryot Microbiol 59:429–514
Ågren GI (2004) The C:N:P stoichiomestry of autotrophs - theory and observations. Ecol Lett 7:185–191
Ågren GI (2008) Stoichiometry and nutrition of plant growth in natural communities. Annu Rev Ecol Syst 39:153–170
Alphei J, Bonkowski M, Scheu S (1996) Protozoa, Nematoda and Lumbricidae in the rhizosphere of Hordelymus europeaus (Poaceae): faunal interactions, response of microorganisms and effects on plant growth. Oecologia 106:111–126
Anderson RV, Coleman DC (1981) Population development and interactions between 2 species of bacteriophagic nematodes. Nematologica 27:6–19
Anderson RV, Elliott ET, McClellan JF, Coleman DC, Cole CV, Hunt HW (1978) Trophic interactions in soils as they affect energy and nutrient dynamics. 3. Biotic interactions of bacteria, amebas, and nematodes. Microb Ecol 4:361–371
Anderson R, Gould W, Woods L, Cambardella C, Ingham R, Coleman D (1983) Organic and inorganic nitrogenous losses by microbivorous nematodes in soil. Oikos 40:75–80
Baath E, Lohm U, Lundgren B, Rosswall T, Soderstrom B, Sohlenius B (1981) Impact of microbial-feeding animals on total soil activity and nitrogen dynamics: a soil microcosm experiment. Oikos 37:257–264
Bardgett RD, Cook R, Yeates GW, Denton CS (1999) The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 212:23–33
Barrios E (2007) Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285
Benizri E, Dedourge O, Dibattista-Leboeuf C, Piutti S, Nguyen C, Guckert A (2002) Effect of maize rhizodeposits on soil microbial community structure. Appl Soil Ecol 21:261–265
Bjornlund L, Rønn R (2008) ‘David and Goliath’ of the soil food web - Flagellates that kill nematodes. Soil Biol Biochem 40:2032–2039
Bjornlund L, Liu MQ, Rønn R, Christensen S, Ekelund F (2012) Nematodes and protozoa affect plants differently, depending on soil nutrient status. Eur J Soil Biol 50:28–31
Blanc C, Sy M, Djigal D, Brauman A, Normand P, Villenave C (2006) Nutrition on bacteria by bacterial-feeding nematodes and consequences on the structure of soil bacterial community. Eur J Soil Biol 42:S70–S78
Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162:617–631
Bonkowski M, Clarholm M (2012) Stimulation of plant growth through interactions of bacteria and protozoa: testing the auxiliary microbial loop hypothesis. Acta Protozool 51:237–247
Bonkowski M, Schaefer M (1997) Interactions between earthworms and soil protozoa: a trophic component in the soil food web. Soil Biol Biochem 29:499–502
Bonkowski M, Griffiths B, Scrimgeour C (2000) Substrate heterogeneity and microfauna in soil organic ‘hotspots’ as determinants of nitrogen capture and growth of ryegrass. Appl Soil 14:37–53
Bonkowski M, Geoghegan IE, Birch ANE, Griffiths BS (2001a) Effects of soil decomposer invertebrates (protozoa and earthworms) on an above-ground phytophagous insect (cereal aphid) mediated through changes in the host plant. Oikos 95:441–450
Bonkowski M, Jentschke G, Scheu S (2001b) Contrasting effects of microbial partners in the rhizosphere: interactions between Norway Spruce seedlings (Picea abies Karst.), mycorrhiza (Paxillus involutus (Batsch) Fr.) and naked amoebae (protozoa). Appl Soil 18:193–204
Bonkowski M, Villenave C, Griffiths B (2009) Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321:213–233
Bonkowski M, Koller R, Jousset AJF (2011) How protozoa structure microbial communities in the rhizosphere of plants. J Phycol 47:S7
Borkott H (1989) Elementgehalte (C, N, P, K) wirbelloser Bodentiere. Z Pflanzenernähr Bodenkd 152:77–80
Bossio DA, Girvan MS, Verchot L, Bullimore J, Borelli T, Albrecht A, Scow KM, Ball AS, Pretty JN, Osborn AM (2005) Soil microbial community response to land use change in an agricultural landscape of western Kenya. Microb Ecol 49:50–62
Brown LK, George TS, Barrett GE, Hubbard SF, White PJ (2013) Interactions between root hair length and arbuscular mycorrhizal colonisation in phosphorus deficient barley (Hordeum vulgare). Plant Soil 372:195–205
Brussaard L (1997) Biodiversity and ecosystem functioning in soil. Ambio 26:563–570
Brussaard L, De Ruiter PC, Brown GG (2007) Soil biodiversity for agricultural sustainability. Agr Ecosyst Environ 121:233–244
Cappellazzo G, Lanfranco L, Fitz M, Wipf D, Bonfante P (2008) Characterization of an amino acid permease from the endomycorrhizal fungus Glomus mosseae. Plant Physiol 147:429–437
Chen X, Liu M, Hu F, Mao X, Li H (2007) Contributions of soil micro-fauna (protozoa and nematodes) to rhizosphere ecological functions. Acta Ecol Sin 27:3132–3143
Cheng YH, Jiang Y, Griffiths BS, Li DM, Hu F, Li HX (2011) Stimulatory effects of bacterial-feeding nematodes on plant growth vary with nematode species. Nematology 13:369–372
Clarholm M (1985a) Interactions of bacteria, protozoa and plants leading to mineralization of soil-nitrogen. Soil Biol Biochem 17:181–187
Clarholm M (1985b) Possible roles for roots, bacteria, protozoa, and fungi in supplying nitrogen to plants. In: Fitter AH (ed) Ecological interactions in soil. Blackwell Scient. Publ., pp 355–365
Cleveland CC, Liptzin D (2007) C: N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Cole CV, Elliott ET, Hunt HW, Coleman DC (1978) Trophic interactions in soils as they affect energy and nutrient dynamics. Phosphorus transformations. Microb Ecol 4:381–387
Coleman DC, Wall DH (2015) Soil fauna: occurrence, biodiversity, and roles in ecosystem function. In: Paul EA (ed) Soil Microbiology, Ecology and Biochemistry, 4th edn. Academic, Boston, pp 111–149
Coleman DC, Cole CV, Anderson RV, Blaha M, Campion MK, Clarholm M, Elliott ET, Hunt HW, Shaefer B, Sinclair J (1977) An analysis of rhizosphere-saprophage interactions in terrestrial ecosystems. Ecol Bull 25:299–309
Coleman DC, Anderson RV, Cole CV, Elliott ET, Woods L, Campion MK (1978) Trophic interactions in soils as they affect energy and nutrient dynamics. Flows of metabolic and biomass carbon. Microb Ecol 4:373–380
Courty PE, Pouysegur R, Buée M, Garbaye J (2006) Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol Biochem 38:1219–1222
Couteaux MM, Darbyshire JF (1998) Functional diversity amongst soil protozoa. Appl Soil 10:229–237
Cowling AJ (1994) Protozoa distribution and adaptation. In: Darbyshire JF (ed) Soil protozoa. CAB International, London, pp 5–42
Crotty FV, Adl SM, Blackshaw RP, Murray PJ (2013) Measuring soil protist respiration and ingestion rates using stable isotopes. Soil Biol Biochem 57:919–921
Darbyshire JF (1994) Soil protozoa. CAB International, London
Darbyshire JF, Davidson MS, Chapman SJ, Ritchie S (1994) Excretion of nitrogen and phosphorus by the soil Ciliate Colpoda steinii when fed the soil bacterium Arthrobacter sp. Soil Biol Biochem 26:1193–1199
De Ley P (1992) The nematode community of a marginal soil at Camberene, Senegal, with special attention to functional morphology and niche partitioning in the family Cephalobidae. Mededelingen van de Koninklijke. Academie voor Wetenschappen, Letteren en Schone Kunsten van België, Klasse der Wetenschappen 53:109–153
De Telegdy‐Kovats L (1932) The growth and respiration of bacteria in sand cultures in the presence and absence of protozoa. Ann Appl Biol 19:65–86
de Vries FT, Thebault E, Liiri M, Birkhofer K, Tsiafouli MA, Bjornlund L, Bracht Jørgensen H, Vincent Brady M, Christensen S, de Ruiter PC, d’Hertefeldt T, Frouz J, Hedlund K, Hemerik L, Gera Hol WH, Hotes S, Mortimer SR, Setala H, Sgardelis SP, Uteseny K, van der Putten WH, Wolters V, Bardgett RD (2013) Soil food web properties explain ecosystem services across European land use systems. Proc Natl Acad Sci U S A 110:14296–14301
Decaëns T (2010) Macroecological patterns in soil communities. Global Ecol Biogeogr 19:287–302
Djigal D, Brauman A, Diop TA, Chotte JL, Villenave C (2004) Influence of bacterial-feeding nematodes (Cephalobidae) on soil microbial communities during maize growth. Soil Biol Biochem 36:323–331
Djigal D, Baudoin E, Philippot L, Brauman A, Villenave C (2010) Shifts in size, genetic structure and activity of the soil denitrifier community by nematode grazing. Eur J Soil Biol 46:112–118
Drevon J-J, Hartwig UA (1997) Phosphorus deficiency increases the argon-induced decline of nodule nitrogenase activity in soybean and alfalfa. Planta 201:463–469
Dupouey J-L, Dambrine E, Laffite J-D, Moares C (2002) Irreversible impact of past land use on forest soils and biodiversity. Ecology 83:2978–2984
Ekelund F (1996) Growth kinetics of five common heterotrophic soil flagellates. Eur J Soil Biol 32:15–24
Ekelund F, Rønn R (1994) Notes on protozoa in agricultural soil with emphasis on heterotrophic flagellates and naked amebas and their ecology. FEMS Microbiol Rev 15:321–353
Ekelund F, Saj S, Vestergard M, Bertaux J, Mikola J (2009) The “soil microbial loop” is not always needed to explain protozoan stimulation of plants. Soil Biol Biochem 41:2336–2342
Elliott E, Cole C, Coleman D, Anderson R, Hunt H, McClellan J (1979) Amoebal growth in soil microcosms: a model system of C, N, and P. Trophic dynamics. Int J Environ Stud 13:169–174
Elliott ET, Anderson RV, Coleman DC, Cole CV (1980) Habitable pore-space and microbial trophic interactions. Oikos 35:327–335
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW (2003) Growth rate–stoichiometry couplings in diverse biota. Ecol Lett 6:936–943
Fanin N, Fromin N, Buatois B, Hattenschwiler S (2013) An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol Lett 16:764–772
Fenner M, Lee W (1989) Growth of seedlings of pasture grasses and legumes deprived of single mineral nutrients. J Appl Ecol 26:223–232
Ferris H, Venette RC, Lau SS (1997) Population energetics of bacterial-feeding nematodes: carbon and nitrogen budgets. Soil Biol Biochem 29:1183–1194
Ferris H, Venette RC, van der Meulen HR, Lau SS (1998) Nitrogen mineralization by bacterial-feeding nematodes: verification and measurement. Plant Soil 203:159–171
Finlay RD (2008) Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59:1115–1126
Foissner W (1999) Soil protozoa as bioindicators: pros and cons, methods, diversity, representative examples. Agric Ecosyst Environ 74:95–112
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Fürst von Lieven A (2003) Functional morphology and evolutionary origin of the three-part pharynx in nematodes. Zoology 106:183–201
Gallet-Budynek A, Brzostek E, Rodgers VL, Talbot JM, Hyzy S, Finzi AC (2009) Intact amino acid uptake by northern hardwood and conifer trees. Oecologia 160:129–138
Gardi C, Jeffery S, Saltelli A (2013) An estimate of potential threats levels to soil biodiversity in EU. Glob Change Biol 19:1538–1548
Geisen S, Fiore-Donno AM, Walochnik J, Bonkowski M (2014) Acanthamoeba everywhere: high diversity of Acanthamoeba in soils. Parasitol Res 113:3151–3158
Geisen S, Tveit AT, Clark IM, Richter A, Svenning MM, Bonkowski M, Urich T (2015) Metatranscriptomic census of active protists in soils. ISME J. doi:10.1038/ismej.2015.30
Glücksman E, Bell T, Griffiths RI, Bass D (2010) Closely related protist strains have different grazing impacts on natural bacterial communities. Environ Microbiol 12:3105–3113
Gould WD, Coleman DC, Rubink AJ (1979) Effect of bacteria and amoebae on rhizosphere phosphatase activity. Appl Environ Microb 37:943–946
Griffiths BS (1994) Soil nutrient flow. In: Darbyshire JF (ed) Soil protozoa. CAB International, Oxford, pp 65–92
Griffiths BS, Ritz K (1988) A technique to extract, enumerate and measure protozoa from mineral soils. Soil Biol Biochem 20:163–173
Griffiths BS, Bonkowski M, Dobson G, Caul S (1999) Changes in soil microbial community structure in the presence of microbial-feeding nematodes and protozoa. Pedobiologia 43:297–304
Griffiths BS, Spilles A, Bonkowski M (2012) C: N: P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol Process 1:1–11
Gusewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hanley M, Fenner M (1997) Seedling growth of four fire‐following Mediterranean plant species deprived of single mineral nutrients. Funct Ecol 11:398–405
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Herdler S, Kreuzer K, Scheu S, Bonkowski M (2008) Interactions between arbuscular mycorrhizal fungi (Glomus intraradices, Glomeromycota) and amoebae (Acanthamoeba castellanii, Protozoa) in the rhizosphere of rice (Oryza sativa). Soil Biol Biochem 40:660–668
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hinsinger P, Brauman A, Devau N, Gerard F, Jourdan C, Laclau JP, Le Cadre E, Jaillard B, Plassard C (2011) Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil 348:29–61
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Horiuchi J-i, Prithiviraj B, Bais HP, Kimball BA, Vivanco JM (2005) Soil nematodes mediate positive interactions between legume plants and rhizobium bacteria. Planta 222:848–857
Ingham RE, Trofymow J, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55:119–140
Irshad U, Villenave C, Brauman A, Plassard C (2011) Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol Biochem 43:2121–2126
Irshad U, Brauman A, Villenave C, Plassard C (2012) Phosphorus acquisition from phytate depends on efficient bacterial grazing, irrespective of the mycorrhizal status of Pinus pinaster. Plant Soil 358:148–161
Jentschke G, Bonkowski M, Godbold DL, Scheu S (1995) Soil protozoa and forest tree growth - Non-nutritional effects and interaction with mycorrhizae. Biol Fert Soils 20:263–269
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Jousset A (2011) Ecological and evolutive implications of bacterial defences against predators. Environ Microbiol 14:1830–1843
Jousset A, Lara E, Wall LG, Valverde C (2006) Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl Environ Microb 72:7083–7090
Jousset A, Scheu S, Bonkowski M (2008) Secondary metabolite production facilitates establishment of rhizobacteria by reducing both protozoan predation and the competitive effects of indigenous bacteria. Funct Ecol 22:714–719
Jousset A, Rochat L, Péchy-Tarr M, Keel C, Scheu S, Bonkowski M (2009) Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J 3:666–674
Jousset A, Rochat L, Scheu S, Bonkowski M, Keel C (2010) Predator–prey chemical warfare determines the expression of biocontrol genes by rhizosphere-associated Pseudomonas fluorescens. Appl Environ Microb 76:5263–5268
Kibblewhite MG (2012) Definition of priority areas for soil protection at a continental scale. Soil Use Manage 28:128–133
Kibblewhite MG, Ritz K, Swift MJ (2008) Soil health in agricultural systems. Philos T Roy Soc B 363:685–701
Koller R, Robin C, Bonkowski M, Ruess L, Scheu S (2013a) Litter quality as driving factor for plant nutrition via grazing of protozoa on soil microorganisms. FEMS Microbiol Ecol 85:241–250
Koller R, Rodriguez A, Robin C, Scheu S, Bonkowski M (2013b) Protozoa enhance foraging efficiency of arbuscular mycorrhizal fungi for mineral nitrogen from organic matter in soil to the benefit of host plants. New Phytol 199:203–211
Koller R, Scheu S, Bonkowski M, Robin C (2013c) Protozoa stimulate N uptake and growth of arbuscular mycorrhizal plants. Soil Biol Biochem 65:204–210
Kreuzer K, Adamczyk J, Iijima M, Wagner M, Scheu S, Bonkowski M (2006) Grazing of a common species of soil protozoa (Acanthamoeba castellanii) affects rhizosphere bacterial community composition and root architecture of rice (Oryza sativa L.). Soil Biol Biochem 38:1665–1672
Krome K, Rosenberg K, Bonkowski M, Scheu S (2009a) Grazing of protozoa on rhizosphere bacteria alters growth and reproduction of Arabidopsis thaliana. Soil Biol Biochem 41:1866–1873
Krome K et al (2009b) Soil bacteria and protozoa affect root branching via effects on the auxin and cytokinin balance in plants. Plant Soil 328:191–201
Kuikman PJ, Van Veen JA (1989) The impact of protozoa on the availability of bacterial nitrogen to plants. Biol Fert Sci 8:13–18
Kuikman PJ, Jansen AG, Van Veen JA, Zehnder AJB (1990) Protozoan predation and the turnover of soil organic-carbon and nitrogen in the presence of plants. Biol Fert Sci 10:22–28
Kuikman PJ, Jansen AG, Van Veen JA (1991) N-15-nitrogen mineralization from bacteria by protozoan grazing at different soil-moisture regimes. Soil Biol Biochem 23:193–200
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Lambshead P (1993) Recent developments in marine benthic biodiversity research. Oceanis 19:5
Lambshead P, Chen Z, Chen W, Chen S, Dickson S (2004) Marine nematode biodiversity. In: Chen ZX et al. (ed) Nematology: Advances and Perspectives. CAB International, pp 436–467
Lavelle P, Spain AV (2001) Soil Ecology. Kluwer, Dordrecht
Leake JR, Johnson D, Donnelly DP, Muckle GE, Boddy L, Read DJ (2004) Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot-Rev Can Bot 82:1016–1045
Lennox LB, Alexander M (1981) Fungicide enhancement of nitrogen fixation and colonization of Phaseolus vulgaris by Rhizobium phaseoli. Appl Environ Microb 41:404–411
Levrat P, Pussard M, Alabouvette C (1992) Enhanced bacterial metabolism of a Pseudomonas strain in response to the addition of culture filtrate of a bacteriophagous amoeba. Eur J Protistol 28:79–84
Louche J, Ali MA, Cloutier-Hurteau B, Sauvage F-X, Quiquampoix H, Plassard C (2010) Efficiency of acid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol Ecol 73:323–335
Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI (2012) Environmental and stoichiometric controls on microbial carbon‐use efficiency in soils. New Phytol 196:79–91
Marmeisse R, Guidot A, Gay G, Lambilliotte R, Sentenac H, Combier JP, Melayah D, Fraissinet-Tachet L, Debaud JC (2004) Hebeloma cylindrosporum - a model species to study ectomycorrhizal symbiosis from gene to ecosystem. New Phytol 163:481–498
Matz C, Kjelleberg S (2005) Off the hook–how bacteria survive protozoan grazing. Trends Microbiol 13:302–307
Mazzola M, de Bruijn I, Cohen MF, Raaijmakers JM (2009) Protozoan-induced regulation of cyclic lipopeptide biosynthesis is an effective predation defense mechanism for Pseudomonas fluorescens. Appl Environ Microb 75:6804–6811
Meier IC, Avis PG, Phillips RP (2013) Fungal communities influence root exudation rates in pine seedlings. FEMS Microbiol Ecol 83:585–595
Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014a) Adjustment of microbial nitrogen use efficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:1–7
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014b) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:1–10
Nasholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Neff JC, Chapin FS III, Vitousek PM (2003) Breaks in the cycle: dissolved organic nitrogen in terrestrial ecosystems. Front Ecol Environ 1:205–211
Neidig N, Jousset A, Nunes F, Bonkowski M, Paul RJ, Scheu S (2010) Interference between bacterial feeding nematodes and amoebae relies on innate and inducible mutual toxicity. Funct Ecol 24:1133–1138
Olsson PA, Baath E, Jakobsen I, Söderstrom B (1996) Soil bacteria respond to presence of roots but not to mycelium of arbuscular mycorrhizal fungi. Soil Biol Biochem 28:463–470
Parry JD (2004) Protozoan grazing of freshwater biofilms. Adv Appl Microbiol 54:167–196
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. Jama-J Am Med Assoc 295:676–680
Pimentel D (2006) Soil erosion: a food and environmental threat. Environ Dev Sust 8:119–137
Plassard C, Dell B (2010) Phosphorus nutrition of mycorrhizal trees. Tree Physiol 30:1129–1139
Plassard C, Bonafos B, Touraine B (2000) Differential effects of mineral and organic N sources, and of ectomycorrhizal infection by Hebeloma cylindrosporum, on growth and N utilization in Pinus pinaster. Plant Cell Environ 23:1195–1205
Plassard C, Louche J, Ali MA, Duchemin M, Legname E, Cloutier-Hurteau B (2011) Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann For Sci 68:33–43
Postma-Blaauw M, de Vries FT, De Goede R, Bloem J, Faber J, Brussaard L (2005) Within-trophic group interactions of bacterivorous nematode species and their effects on the bacterial community and nitrogen mineralization. Oecologia 142:428–439
Puglisi E, Pascazio S, Suciu N, Cattani I, Fait G, Spaccini R, Crecchio C, Piccolo A, Trevisan M (2013) Rhizosphere microbial diversity as influenced by humic substance amendments and chemical composition of rhizodeposits. J Geochem Explor 129:82–94
Pussard M, Alabouvette C, Levrat P (1994) Protozoan interactions with the soil microflora and possibilities for biocontrol of plant pathogens. In: Darbyshire JF (ed) Soil Protozoa. CAB International, Oxford, pp 123–146
Ramirez C, Alexander M (1980) Evidence suggesting protozoan predation on Rhizobium associated with germinating seeds and in the rhizosphere of beans (Phaseolus vulgaris L.). Appl Environ Microb 40:492–499
Rønn R, McCaig AE, Griffiths BS, Prosser JI (2002) Impact of protozoan grazing on bacterial community structure in soil microcosms. Appl Environ Microb 68:6094–6105
Rønn R, Vestergard M, Ekelund F (2012) Interactions between bacteria, protozoa and nematodes in soil. Acta Protozool 51:223–235
Rosenberg K, Bertaux J, Krome K, Hartmann A, Scheu S, Bonkowski M (2009) Soil amoebae rapidly change bacterial community composition in the rhizosphere of Arabidopsis thaliana. ISME J 3:675–684
Saleem M, Fetzer I, Dormann CF, Harms H, Chatzinotas A (2012) Predator richness increases the effect of prey diversity on prey yield. Nat Commun 3:1305
Sardans J, Rivas-Ubach A, Penuelas J (2012) The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111:1–39
Schlaghamersky J, Eisenhauer N, Frelichc LE (2014) Earthworm invasion alters enchytraeid community composition and individual biomass in northern hardwood forests of North America. Appl Soil 83:159–169
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16:930–939
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Somasundaram S, Bonkowski M, Iijima M (2008) Functional role of mucilage-border cells: a complex facilitating protozoan effects on plant growth. Plant Prod Sci 11:344–351
Sundin P, Valeur A, Olsson S, Odham G (1990) Interactions between bacteria-feeding nematodes and bacteria in the rape rhizosphere - Effects on root exudation and distribution of bacteria. FEMS Microbiol Ecol 73:13–22
Tao J, Chen X, Liu M, Hu F, Griffiths B, Li H (2009) Earthworms change the abundance and community structure of nematodes and protozoa in a maize residue amended rice-wheat rotation agro-ecosystem. Soil Biol Biochem 41:898–904
Tibbett M, Sanders FE (2002) Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Ann Bot-London 89:783–789
Venette RC, Ferris H (1998) Influence of bacterial type and density on population growth of bacterial-feeding nematodes. Soil Biol Biochem 30:949–960
Vitousek PM et al (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57:1–45
Wagg C, Bender SF, Widmer F, van der Heijden MGA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci U S A 111:5266–5270
Wall DH, Bardgett RD, Kelly EF (2010) Biodiversity in the dark. Nat Geosci 3:297–298
Wallenda T, Read DJ (1999) Kinetics of amino acid uptake by ectomycorrhizal roots. Plant Cell Environ 22:179–187
Weekers PH, Bodelier PL, Wijen JP, Vogels GD (1993) Effects of grazing by the free-living soil amoebae Acanthamoeba castellanii, Acanthamoeba polyphaga, and Hartmannella vermiformis on various bacteria. Appl Environ Microb 59:2317–2319
Weisse T (2002) The significance of inter- and intraspecific variation in bacterivorous and herbivorous protists. Anton Leeuw Int J G 81:327–341
Wright DJ (1975) Elimination of nitrogenous compounds by Panagrellus redivivus, Goodey, 1945 (Nematoda: Cephalobidae). Comp Biochem Phys B 52:247–253
Xiao HF, Griffiths B, Chen XY, Liu MQ, Jiao JG, Hu F, Li HX (2010) Influence of bacterial-feeding nematodes on nitrification and the ammonia-oxidizing bacteria (AOB) community composition. Appl Soil 45:131–137
Xu X, Thornton PE, Post WM (2013) A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Global Ecol Biogeogr 22:737–749
Yeates GW (2003) Nematodes as soil indicators: functional and biodiversity aspects. Biol Fert Sci 37:199–210
Yeates G (2007) Abundance, diversity, and resilience of nematode assemblages in forest soils. Can J Forest Res 37:216–225
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol Monogr 85:133–155
Zwart KB, Darbyshire JF (1992) Growth and nitrogenous excretion of a common soil flagellate Spumella sp. - a laboratory experiment. J Soil Sci 43:145–157
Acknowledgments
We thank Claire Marsden for providing helpful comments on an earlier draft of this review. We would like to thank the reviewers for their insightful comments on the paper, as these comments led us to an improvement of the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Hinsinger.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Trap, J., Bonkowski, M., Plassard, C. et al. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 398, 1–24 (2016). https://doi.org/10.1007/s11104-015-2671-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2671-6