Abstract
Background and aims
The biomechanics of root systems influence plant lodging resistance and soil structural stabilisation. Tissue age has the potential to influence root biomechanical properties through changes in cell wall chemistry, root anatomy and morphology. Within a root system the internal structures of roots are known to vary markedly within different root types. Nodal, seminal and lateral roots of Barley (Hordeum vulgare) have differing biomechanical behaviour in tension. This study examines the effects of root age on biomechanical properties of barley root types (Hordeum vulgare) under abiotic stress.
Methods
Root age was determined as a function of the distance from root tip with abiotic stresses consisting of waterlogging and restriction to root elongation rate through increased soil bulk density. Linear regression analyses were performed on log-transformed tensile strength and Young’s modulus data with best fits determined for single and multiple parameter models to root morphological properties.
Results
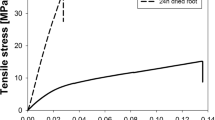
Regression co-efficients and Akaike values showed that distance from root tip (taken as a proxy of root age) was the best single variable for prediction of both root tensile strength and Young’s modulus. Incorporation of both distance from root tip and root diameter and root type increased the reliability of predictions for root biomechanical properties from 47 to 57 % for tensile strength and 35 to 62 % for Young’s modulus.
Conclusions
The age effect may partly explain some scatter in both Young’s modulus and tensile strength to diameter relationship, commonly cited in the literature.



Similar content being viewed by others
References
Beek L, Wint J, Cammeraat L, Edwards J (2005) Observation and simulation of root reinforcement on abandoned Mediterranean slopes. Plant and Soil 278:55–74
Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot 57:437–447
Berry PM, Sterling M, Spink JH, Baker CJ, Sylvester-Bradley R, Mooney SJ, Tams AR, Ennos AR (2004) Understanding and reducing lodging in cereals. Elsevier Academic Press Inc, San Diego
Berry PM, Sterling M, Mooney SJ (2006) Development of a model of lodging for barley. J Agron Crop Sci 192:151–158
Bingham IJ (2007) Quantifying the presence and absence of turgor for the spatial characterization of cortical senescence in roots of Triticum aestivum (Poaceae). Am J Bot 94:2054–2058
Bischetti GB, Chiaradia EA, Simonato T, Speziali B, Vitali B, Vullo P, Zocco A (2005) Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant and Soil 278:11–22
Blacklow WM (1972) Influence of temperature on germination and elongation of the radicle and shoot of corn (Zea mays L.). Crop Sci 12:647–650
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Campbell MM, Sederoff RR (1996) Variation in lignin content and composition - Mechanism of control and implications for the genetic improvement of plants. Plant Physiol 110:3–13
Coppin NJ, Richards IG (1990) Use of vegetation in civil engineering. Butterworths, London
Dumlao MR, Goyal V, Ramananarivo S, DeJong J, Waller J, Silk WK (2012) Role of root development in conferring soil strength. International Society of Root Research conference, Dundee
Easson DL, Pickles SJ, White EM (1995) A study of the tensile force required to pull wheat roots from soil. Ann Appl Biol 127:363–373
Ellis FB, Barnes BT (1983) Growth and development of root systems of winter cereals grown after different tillage methods including direct drilling. Plant and Soil 55:283–295
Galamay TO, Yamauchi A, Nonoyama T, Kono Y (1992) Acropetal lignification in protective tissues of cereal nodal root axes as affected by different soil-moisture conditions. Jpn J Crop Sci 61:511–517
Garthwaite AJ, von Bothmer R, Colmer TD (2003) Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct Plant Biol 30:875–889
Genet M, Stokes A, Salin F, Mickovski S, Fourcaud T, Dumail JF, van Beek R (2005) The influence of cellulose content on tensile strength in tree roots. Plant and Soil 278:1–9
Genet M, Kokutse N, Stokes A, Fourcaud T, Cai X, Ji J, Mickovski S (2008) Root reinforcement in plantations of Cryptomeria japonica D. Don: effect of tree age and stand structure on slope stability. For Ecol Manage 256:1517–1526
Gibson LJ (2012) The hierarchical structure and mechanics of plant materials. J R Soc Interface 9:2749–2766
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Gray DH, Ohashi H (1983) Mechanics of fiber reinforcement in sand. J Geotech Eng ASCE 109:335–353
Gregory PJ (1986) Response to temperature in a stand of pearl millet (Pennisetum typhoides S. & H.) VIII. Root growth. J Exp Bot 37:379–388
Hales TC, Ford CR, Hwang T, Vose JM, Band LE (2009) Topographic and ecologic controls on root reinforcement. J Geophys Res Earth Surf 114:F03013
Hallett PD, Feeney DS, Bengough AG, Rillig MC, Scrimgeour CM, Young IM (2009) Disentangling the impact of AM fungi versus roots on soil structure and water transport. Plant and Soil 314:183–196
Hathaway R, Penny D (1975) Root strength in some Populus and Salix clones. N Z J Bot 13:333–344
Jaffe MJ (1973) Thigmomorphogenesis - Response of Plant-Growth and Development to Mechanical Stimulation - with Special Reference to Bryonia-Dioica. Planta 114:143–157
Kotula L, Ranathunge K, Schreiber L, Steudle E (2009) Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot 60:2155–2167
Loades KW, Bengough AG, Bransby MF, Hallett PD (2010) Planting density influence on fibrous root reinforcement of soils. Ecol Eng 36:276–284
Loades KW, Bengough AG, Bransby MF, Hallett PD (2013) Biomechanics of nodal, seminal and lateral roots of barley: effects of diameter, waterlogging and mechanical impedance. Plant and Soil 370(1-2):407–418
Mao Z, Saint-André L, Genet M, Mine F-X, Jourdan C, Rey H, Courbaud B, Stokes A (2012) Engineering ecological protection against landslides in diverse mountain forests: Choosing cohesion models. Ecol Eng 45:55–69
Mattia C, Bischetti GB, Gentile F (2005) Biotechnical characteristics of root systems of typical Mediterranean species. Plant and Soil 278:23–32
Mickovski S, Hallett PD, Bransby MF, Davies MCR, Sonnenberg R, Bengough AG (2009) Mechanical reinforcement of soil by willow roots: impacts of root properties and root failure mechanism. Soil Sci Soc Am J 73:1276–1285
Pellerin S, Pages L (1994) Evaluation of parameters describing the root system architecture of field grown maize plants (Zea mays L.) I. Elongation of seminal and nodal roots and extension of their branched zone. Plant and Soil 164:155–167
Pollen N (2007) Temporal and spatial variability in root reinforcement of streambanks: Accounting for soil shear strength and moisture. CATENA 69:197–205
Pollen N, Simon A (2005) Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resour Res 41:W07025
Pregitzer KS (2002) Fine roots of trees - a new perspective. New Phytol 154:267–270
Rose DA (1983) The description of the growth of root systems. Plant and Soil 75:405–415
Stokes A, Atger C, Bengough AG, Fourcaud T, Sidle RC (2009) Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant and Soil 324:1–30
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Thomas FM, Molitor F, Werner W (2014) Lignin and cellulose concentrations in roots of Douglas fir and European beech of different diameter classes and soil depths. Trees Struct Funct 28:309–315
Waldron L, Dakessian S (1981) Soil reinforcement by roots: calculation of increased soil shear resistance from root properties. Soil Sci 132:427–435
Watt M, McCully ME, Kirkegaard JA (2003) Soil strength and rate of root elongation alter the accumulation of Pseudomonas spp. and other bacteria in the rhizosphere of wheat. Funct Plant Biol 30:482–491
Watt M, Silk WK, Passioura JB (2006) Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Ann Bot 97:839–855
White NA, Hallett PD, Feeney D, Palfreyman JW, Ritz K (2000) Changes to water repellence of soil caused by the growth of white-rot fungi: studies using a novel microcosm system. FEMS Microbiol Lett 184:73–77
Zeier J, Ruel K, Ryser U, Schreiber L (1999) Chemical analysis and immunolocalisation of lignin and suberin in endodermal and hypodermal/rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta 209:1–12
Acknowledgments
The James Hutton Institute receives funding from the Scottish Government. The authors would also like to thank Jim McNicol from Biomathematics and Statistics Scotland for his advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alexia Stokes.
Rights and permissions
About this article
Cite this article
Loades, K.W., Bengough, A.G., Bransby, M.F. et al. Effect of root age on the biomechanics of seminal and nodal roots of barley (Hordeum vulgare L.) in contrasting soil environments. Plant Soil 395, 253–261 (2015). https://doi.org/10.1007/s11104-015-2560-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2560-z