Abstract
Aims
Subsoil organic carbon (OC) tends to be older and is presumed to be more stable than topsoil OC, but the reasons for this are not yet resolved. One hypothesis is that decomposition rates decrease with increasing soil depth. We tested whether decomposition rates of beech fine root litter varied with depth for a range of soils using a litterbag experiment in German beech forest plots.
Methods
In three study regions (Schorfheide-Chorin, Hainich-Dün and Schwäbische-Alb), we buried 432 litterbags containing 0.5 g of standardized beech root material (fine roots with a similar chemical composition collected from 2 year old Fagus sylvatica L. saplings, root diameter < 2 mm) at three different soil depths (5, 20 and 35 cm). The decomposition rates as well as the changes in the carbon (C) and nitrogen (N) concentrations of the decomposing fine root litter were determined at a 6 months interval during a 2 years field experiment.
Results
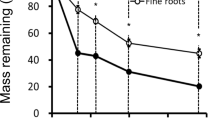
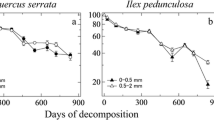
The amount of root litter remaining after 2 years of field incubation differed between the study regions (76 ± 2 % in Schorfheide-Chorin, 85 ± 2 % in Schwäbische-Alb, and 88 ± 2 % in Hainich-Dün) but did not vary with soil depth.
Conclusions
Our results indicate that the initial fine root decomposition rates are more influenced by regional scale differences in environmental conditions including climate and soil parent material, than by changes in microbial activities with soil depth. Moreover, they suggest that a similar potential to decompose new resources in the form of root litter exists in both surface and deep soils.



Similar content being viewed by others
References
Berg B (1984) Decomposition of root litter and some factors regulating the process: long-term root litter decomposition in a Scots pine forest. Soil Biol Biochem 16:609–617. doi:10.1016/0038-0717(84)90081-6
Birkhofer K, Schöning I, Alt F, Herold N, Klarner B, Maraun M, Marhan S, Oelmann Y, Wubet T, Yurkov A, Begerow D, Berner D, Buscot F, Daniel R, Diekötter T, Ehnes RB, Erdmann G, Fischer C, Foesel B, Groh J, Gutknecht J, Kandeler E, Lang C, Lohaus G, Meyer A, Nacke H, Näther A, Overmann J, Polle A, Pollierer MM, Scheu S, Schloter M, Schulze ED, Schulze W, Weinert J, Weisser WW, Wolters V, Schrumpf M (2012) General relationships between abiotic soil properties and soil biota across spatial scales and different land-Use types. PLoS ONE 7(8), e43292. doi:10.1371/journal.pone.0043292
Brunner I, Godbold DL (2007) Tree roots in a changing world. J For Res 12:78–82. doi:10.1007/s10310-006-0261-4
Chen H, Harmon ME, Griffiths RP, Hicks W (2000) Effects of temperature and moisture on carbon respired from decomposing woody roots. For Ecol Manag 138:51–64. doi:10.1016/S0378-1127(00)00411-4
Dornbush ME, Isenhart TM, Raich JW (2002) Quantifying fine-root decomposition an alternative to buried litterbags. Ecology 83:2985–2990
Dioumaeva I, Trumbore S, Schuur EAG, Goulden ML, Litvak M, Hirsch AI (2002) Decomposition of peat from upland boreal forest: temperature dependence and sources of respired carbon. J Geophys Res: Atmos 107:8222. doi:10.1029/2001jd000848
Dixon RK, Solomon A, Brown S, Houghton R, Trexier M, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Fierer N, Allen AS, Schimel JP, Holden PA (2003a) Controls on microbial CO2 production: a comparison of surface and subsurface soil horizons. Glob Chang Biol 9:1322–1332
Fierer N, Schimel JP, Holden PA (2003b) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176. doi:10.1016/S0038-0717(02)00251-1
Fischer M, Bossdorf O, Gockel S, Hänsel F, Hemp A, Hessenmöller D, Korte G, Nieschulze J, Pfeiffer S, Prati D, Renner S, Schöning I, Schumacher U, Wells K, Buscot F, Kalko EKV, Linsenmair KE, Schulze ED, Weisser WW (2010) Implementing large-scale and long-term functional biodiversity research: the biodiversity exploratories. Basic Appl Ecol 11:473–485. doi:10.1016/j.baae.2010.07.009
Fontaine S, Barot S, Barre P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280, http://www.nature.com/nature/journal/v450/n7167/suppinfo/nature06275_S1.html
Gill R, Burke I (2002) Influence of soil depth on the decomposition of bouteloua gracilis roots in the shortgrass steppe. Plant Soil 241:233–242. doi:10.1023/a:1016146805542
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat D (2010) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monogr 81:89–102
Grüneberg E, Schöning I, Kalko EKV, Weisser WW (2010) Regional organic carbon stock variability: a comparison between depth increments and soil horizons. Geoderma 155:426–433. doi:10.1016/j.geoderma.2010.01.002
Handa IT, Aerts R, Berendse F, Berg MP, Bruder A, Butenschoen O, Chauvet E, Gessner MO, Jabiol J, Makkonen M, McKie BG, Malmqvist B, Peeters ETHM, Scheu S, Schmid B, van Ruijven J, Vos VCA, Hattenschwiler S (2014) Consequences of biodiversity loss for litter decomposition across biomes. Nature 509:218–221. doi:10.1038/nature13247
Heim A, Frey B (2004) Early stage litter decomposition rates for Swiss forests. Biogeochemistry 70:299–313. doi:10.1007/s10533-003-0844-5
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Herold N, Schöning I, Berner D, Haslwimmer H, Kandeler E, Michalzik B, Schrumpf M (2014) Vertical gradients of potential enzyme activities in soil profiles of European beech, Norway spruce and Scots pine dominated forest sites. Pedobiologia 7:181–189
Hishi T (2007) Heterogeneity of individual roots within the fine root architecture: casual links between physiological and ecosystem functions. J For Res 12:126–133
Hobbie S, Oleksyn J, Eissenstat D, Reich P (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513. doi:10.1007/s00442-009-1479-6
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436. doi:10.2307/2641104
Klotzbücher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011) A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92:1052–1062. doi:10.1890/10-1307.1
Li A, Fahey TJ, Pawlowska TE, Fisk MC, Burtis J (2015) Fine root decomposition, nutrient mobilization and fungal communities in a pine forest ecosystem. Soil Biol Biochem 83:76–83
Mendez-Millan M, Dignac MF, Rumpel C, Rasse DP, Derenne S (2010) Molecular dynamics of shoot vs. root biomarkers in an agricultural soil estimated by natural abundance 13C labelling. Soil Biol Biochem 42:169–177. doi:10.1016/j.soilbio.2009.10.010
Mikan CJ, Schimel JP, Doyle AP (2002) Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol Biochem 34:1785–1795. doi:10.1016/S0038-0717(02)00168-2
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364. doi:10.1126/science.1134853
Paul EA, Follett RF, Leavitt SW, Halvorson A, Peterson GA, Lyon DJ (1997) Radiocarbon dating for determination of soil organic matter pool sizes and dynamics. Soil Sci Soc Am J 61:1058–1067. doi:10.2136/sssaj1997.03615995006100040011x
Qualls RG, Haines BL (1992) Biodegradability of dissolved organic matter in forest throughfall, soil solution, and stream water. Soil Sci Soc Am J 56:578–586
Raessler M, Rothe J, Hilke I (2005) Accurate determination of Cd, Cr, Cu and Ni in woodlice and their skins—is moulting a means of detoxification? Sci Total Environ 337:83–90. doi:10.1016/j.scitotenv.2004.07.008
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. doi:10.1007/s11104-004-0907-y
Rovira P, Ramón Vallejo V (2002) Mineralization of carbon and nitrogen from plant debris, as affected by debris size and depth of burial. Soil Biol Biochem 34:327–339. doi:10.1016/S0038-0717(01)00186-9
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158. doi:10.1007/s11104-010-0391-5
Sanaullah M, Chabbi A, Leifeld J, Bardoux G, Billou D, Rumpel C (2011) Decomposition and stabilization of root litter in top- and subsoil horizons: what is the difference? Plant Soil 338:127–141. doi:10.1007/s11104-010-0554-4
Schlesinger WH (1997) Biogeochemistry: an analysis of global change. Academic Press, San Diego
Schöning I, Kögel-Knabner I (2006) Chemical composition of young and old carbon pools throughout Cambisol and Luvisol profiles under forests. Soil Biol Biochem 38:2411–2424. doi:10.1016/j.soilbio.2006.03.005
Schrumpf M, Kaiser K, Guggenberger G, Persson T, Kögel-Knabner I, Schulze ED (2013) Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10:1675–1691. doi:10.5194/bg-10-1675-2013
Solly EF, Schöning I, Boch S, Kandeler E, Marhan S, Michalzik B, Müller J, Zscheischler J, Trumbore SE, Schrumpf M (2014) Factors controlling decomposition rates of fine root litter in temperate forests and grasslands. Plant Soil 382:203–218. doi:10.1007/s11104-014-2151-4
Sun T, Mao Z, Dong L, Hou L, Song Y, Wang X (2013) Further evidence for slow decomposition of very fine roots using two methods: litterbags and intact cores. Plant Soil 366:633–646. doi:10.1007/s11104-012-1457-3
Thoms C, Gattinger A, Jacob M, Thomas FM, Gleixner G (2010) Direct and Indirect efects of tree diversity drive soil microbial diversity in temperate deciduous forest. Soil Biol Biochem 42:1558–1565
Trumbore S (2000) Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecol Appl 10:399–411
Trumbore S (2009) Radiocarbon and soil carbon dynamics. Annu Rev Earth Planet Sci 37:47–66
van Huysen TL, Harmon ME, Perakis SS, Chen H (2013) Decomposition and nitrogen dynamics of 15 N-labeled leaf, root, and twig litter in temperate coniferous forests. Oecologia 173:1563–1573
Weaver JE (1947) Rate of decomposition of roots and rhizomes of certain range grasses in undisturbed prairie soil. Ecology 28:221–240
Weaver JE, Hougen V, Weldon M (1935) Relation of root distribution to organic matter in prairie soil. Bot Gaz 96:389–420
Yang H, Yan R, Chen H, Zheng C, Lee DH, Liang DT (2005) In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuel 20:388–393. doi:10.1021/ef0580117
Acknowledgments
We thank Steffen Both, Jörg Hailer and Uta Schumacher for their help with the field work and technical organization. The authors acknowledge Beate Michalzik and Carlos Sierra for their valuable comments on the manuscript. We thank Theresa Klötzing for technical support, and Ines Hilke and Birgit Fröhlich for the CN analysis. Jakob Zscheischler for the mathematical and statistical help. We thank the managers of the three Exploratories, Kirsten Reichel-Jung, Swen Renner, Katrin Hartwich, Sonja Gockel, Kerstin Wiesner, and Martin Gorke for their work in maintaining the plot and project infrastructure; Christiane Fischer and Simone Pfeiffer for giving support through the central office, Michael Owonibi for managing the central data base, and Markus Fischer, Eduard Linsenmair, Dominik Hessenmöller, Jens Nieschulze, Daniel Prati, François Buscot, Ernst-Detlef Schulze, Wolfgang W. Weisser and the late Elisabeth Kalko for their role in setting up the Biodiversity Exploratories project. The work has been funded by the DFG Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” (SCHR 1181/2-1) and the Max-Planck-Society. Field work permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG). Emily Solly conducted this work as part of the International Max Planck Research School for Global Biogeochemical Cycles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Kees Jan van Groenigen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S5
Mean± SD of soil pH, soil texture and enzyme activities, in different soil horizons in the three study regions (data from Herold et al. (2014)). Significant differences between study regions are indicated by capital letters and between soil depths by lowercase letters according to Tukey HSD test (p < 0.05). Abbreviations: BG=ß-glucosidase activity, NAG=N-acetylglucosaminidase activity, Phos=phosphatase activity. (DOC 886 kb)
Rights and permissions
About this article
Cite this article
Solly, E.F., Schöning, I., Herold, N. et al. No depth-dependence of fine root litter decomposition in temperate beech forest soils. Plant Soil 393, 273–282 (2015). https://doi.org/10.1007/s11104-015-2492-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2492-7