Abstract
Aims
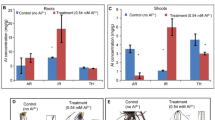
The root-to-shoot transport of manganese (Mn) exhibited intra-specific characters in different grape genotypes. The majority of Mn was stored in the roots of the grape cultivar Jinshou, while it was mainly transferred to the shoots in the cultivar Combier. The aims of the present study was to reveal the complex interplay of gene expression endowing grape a high tolerance to excess Mn and to explore the relation of the expression of Mn transporters with the contrast root-to-shoot translocation pattern of excess Mn in different grape cultivars.
Methods
The root transcriptome changes in both cultivars were analyzed by high-throughput sequencing and validated by quantitative RT-PCR.
Results and conclusion
Compared to Jinshou, Combier exhibited a markedly high transcripts level in the Mn transporter unigenes in the roots independent of the Mn treatment, accompanied by a higher expression level of genes encoding nicotianamine synthase, heavy metal-transporting ATPase, ZIP family member and IRT1-like proteins, which could facilitate Mn transport from the roots to the shoots in Combier. However, the expression level of genes involved in the subcellular vesicular transport pathway was much higher in Jinshou than in Combier, with a higher transcripts level of V-ATPase, vacuolar protein and the proteins for the synthesis of organic acid, such as the citrate cycle and glycolysis pathway. All of these changes allowed Mn to be easily chelated and compartmented to root cortical and epidermic cell vacuoles in Jinshou, accompanied with higher transcription and activity levels of stress-related enzymes, endowing Jinshou a high degree of tolerance to excess Mn. The grape transcriptome responses to Mn stress were also discussed.






Similar content being viewed by others
Abbreviations
- Mn:
-
Manganese
- DEG:
-
Differentially expressed gene
- DGE:
-
Digital gene expression
- TPM:
-
Number of transcripts per million clean tags
- PCR:
-
Polymerase chain reaction
- qRT-PCR:
-
Quantitative real time polymerase chain reaction
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- POD:
-
Guaiacol peroxidase
- SOD:
-
Superoxide dismutase
- CK:
-
The control
- MDA:
-
Malondialdehyde
- BTC:
-
Biological transfer coefficient
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- IRT:
-
Iron-regulated transporter
- MT:
-
Metallothioneins
- HMA:
-
Heavy metal-transporting P-type ATPase
- NAS:
-
Nicotianamine synthase
- MTP:
-
Metal tolerance protein
References
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7:986–995
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bidwell SD, Batianoff GN, Woodrow IE, Sommer-Knudsen J (2002) Hyperaccumulation of manganese in the rainforest tree Austromyrtus bidwillii (Myrtaceae) from Queensland, Australia. Funct Plant Biol 29:899–905
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cailliatte R, Schikora A, Briat JF, Mari S, Curie C (2010) High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22:904–917
Chakraborty D, Kumar SA, Sen M, Apte S, Das S, Acharya R, Das T, Reddy A, Roychaudhury S, Rajaram H (2011) Manganese and iron both influence the shoot transcriptome of Typha angustifolia despite distinct preference towards manganese accumulation. Plant Soil 342:301–317
Clemensson-Lindell A (1994) Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: applications and limitations. Plant Soil 159:297–300
Dietz KJ, Tavakoli N, Kluge C, Mimura T, Sharma SS, Harris GC (2001) Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J Exp Bot 52:1969–1980
Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Krämer U (2004) Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J 39:425–439
Dučić T, Polle A (2007) Manganese toxicity in two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply. Funct Plant Biol 34:31–40
Dučić T, Leinemann L, Finkeldey R, Polle A (2006) Uptake and translocation of manganese in seedlings of two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca). New Phytol 17:11–20
Fernando D, Mizuno T, Woodrow I, Baker A, Collins R (2010) Characterization of foliar manganese (Mn) in Mn (hyper) accumulators using X-ray absorption spectroscopy. New Phytol 188:1014–1027
Foy CD (1984) Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil. In: Adams F (ed.) Soil acidity and liming. 2nd ed. Amer soc agron crop sci Soc Amer, and Soil Sci Soc Amer. Madison, Wisconsin, pp 57–97
Guo WJ, Bundithya W, Goldsbrough PB (2003) Characterization of the Arabidopsis metallothionein gene family: tissue-specific expression and induction during senescence and in response to copper. New Phytol 159:369–381
Hall J (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophy 125:189–198
Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J et al (2004) P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16:1327–1339
Iandolino A, Nobuta K, da Silva FG, Cook DR, Meyers BC (2008) Comparative expression profiling in grape (Vitis vinifera) berries derived from frequency analysis of ESTs and MPSS signatures. BMC Plant Biol 8:53
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T et al (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45:335–346
Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M et al (2010) Rice metal-nicotianamine transporter, OsYSL2, is required for long distance transport of iron and manganese. Plant J 62:379–390
Jacobson HGM, Swanback TR (1932) Manganese content of certain Connecticut soils and its relation to the growth of tobacco. J Am Soc Agron 24:237–245
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40:37–44
Li R, Li Y, Kristiansen K, Wang J (2008) SOAP: short oligonucleotide alignment program. Bioinformatics 24:713–714
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 25:402–408
Macfarlane G, Burchett M (2001) Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar Poll Bull 42:233–240
Memon AR, Yatazawa M (1984) Nature of manganese complexes in manganese accumulator plant-Acanthopanax sciadophylloides. J Plant Nutr 7:961–974
Milner MJ, Seamon J, Craft E, Kochian LV (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J Exp Bot 64:369–381
Morris HD, Pierre WH (1949) Minimum concentrations of manganese necessary for injury to various legumes in culture solutions. Agron J 41:107–112
Morrissy AS, Morin RD, Delaney A, Zeng T, McDonald H, Jones S, Zhao Y, Hirst M, Marra MA (2009) Next-generation tag sequencing for cancer gene expression profiling. Genome Res 19:1825–1835
Mou D, Yao Y, Yang Y, Zhang Y, Tian C, Achal V (2011) Plant high tolerance to excess manganese related with root growth, manganese distribution and antioxidative enzyme activity in three grape cultivars. Ecotox Environ Saf 74:776–786
Najeeb U, Xu L, Ali S, Jilani G, Gong HJ, Shen WQ (2009) Citric acid enhances the phytoextraction of manganese and plant growth by alleviating the ultrastructural damages in Juncus effusus L. J Hazard Mat 170:1156–1163
Page V, Feller U (2005) Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Annals Bot 96:425–434
Pan Z, Zeng Y, An J, Ye J, Xu Q, Deng X (2012) An integrative analysis of transcriptome and proteome provides new insights into carotenoid biosynthesis and regulation in sweet orange fruits. J Proteomics 75:2670–2684
Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S (2008) Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT11. Plant Physiol 148:455–466
Pittman JK (2005) Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol 167:733–742
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic, New York, pp 685–690
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes (salicylic acid-mediated oxidative damage requires H2O2). Plant Physiol 115:137–149
Sasaki A, Yamaji N, Yokosho K, Ma JF (2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24:2155–2167
Singh KB, Foley RC, Oñate-Sánchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5:430–436
Stephan UW, Scholz G (1993) Nicotianamine: mediator of transport of iron and heavy metals in the phloem? Physiol Plant 88:522–529
Takahashi R, Bashir K, Ishimaru Y, Nishizawa NK, Nakanishi H (2012) The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Sign Beh 7:1605–1607
Thapa G, Sadhukhan A, Panda SK, Sahoo L (2012) Molecular mechanistic model of plant heavy metal tolerance. BioMetals 25:489–505
Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ 37:140–152
Turchi A, Tamantini I, Camussi AM, Racchi ML (2012) Expression of a metallothionein A1 gene of Pisum sativum in white poplar enhances tolerance and accumulation of zinc and copper. Plant Sci 183:50–56
Tüzen M (2003) Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem J:289–297
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Vert G, Barberon M, Zelazny E, Séguéla M, Briat JF, Curie C (2009) Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta 229:1171–1179
Wang X, Liu Y, Zeng G, Chai L, Xiao X, Song X, Min Z (2008) Pedological characteristics of Mn mine tailings and metal accumulation by native plants. Chemosphere 72:1260–1266
Weber M, Trampczynska A, Clemens S (2006) Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ 29:950–963
Xu X, Shi J, Chen X, Chen Y, Hu T (2009) Chemical forms of manganese in the leaves of manganese hyperaccumulator Phytolacca acinosa Roxb. (Phytolaccaceae). Plant Soil 318:197–204
Yang M, Zhang W, Dong H, Zhang Y, Lv K, Wang D, Lian X (2013) OsNRAMP3 is a vascular bundles-specific manganese transporter that is responsible for manganese distribution in rice. PLoS ONE 8:e83990
Yao Y, Xu G, Mou D, Wang J, Ma J (2012a) Subcellular Mn compartation, anatomic and biochemical changes of two grape varieties in response to excess manganese. Chemosphere 89:150–157
Yao YA, Wang J, Ma X, Lutts S, Sun C, Ma J, Yang Y, Achal V, Xu G (2012b) Proteomic analysis of Mn-induced resistance to powdery mildew in grape. J Exp Bot 63:5155–5170
Yao YA, Mou D, Xu G, Lutts S, Achal V, Ma J (2012c) Contrasting performance and different tolerance of chestnut rose and grape to excess manganese. J Plant Growth Regul 31:416–426
Zhou ZS, Zeng HQ, Liu ZP, Yang ZM (2012) Genome-wide identification of Medicago truncatula microRNAs and their targets reveals their differential regulation by heavy metal. Plant Cell Environ 35:86–99
Acknowledgments
This research was supported by the talent program of Southwestern University of Science and Technology (No. 13zx7116) as well as the foundation of Sichuan Engineering Center for Biomass Resource exploitation and modification (No. 12zxsk10), Youth foundation of science and technology in Sichuan (No. No.2014JQ0016) and Project of Innovation research team in Sichuan Education Administration (No. 13TD0023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Saturation analysis of MPSS sequencing; a and b separately show the trend of sequence saturation of plant roots in the control and Mn-treated Combier; c and d separately show the trend of sequence saturation in the control and Mn treated Jinshou (GIF 56 kb)
Fig. S2
Distribution of the total and distinct tags in the root libraries of two grapevine cultivars that were treated or not with Mn. a, c, e and g separately show the distribution of, respectively, the total tags in the CK of Combier, the Mn treatment of Combier, the CK of Jinshou, and the Mn treatment of Jinshou; b, d, f and h separately show the distribution of, respectively, the distinct total tags in the CK of Combier, the Mn treatment of Combier, the CK of Jinshou, and the Mn treatment of Jinshou (GIF 89 kb)
Fig. S3
Comparison of transcript expression of different genes in the roots between two grapevine cultivars under control or Mn treatment conditions using the qRT-PCR and DGE techniques. The relative gene expression as measured by qRT-PCR was evaluated using the comparative cycle threshold method using Actin 1 as the reference gene. C-CK, control of Combier; C-Mn, Mn treatment of Combier; J-CK, control of Jinshou; J-Mn, Mn treatment of Jinshou. (GIF 120 kb)
Fig. S4
Schematic diagram of the transcript changes of plant roots in the KEGG pathway between two grapevine cultivars under Mn-treated or control conditions (C-CK vs. J-CK; C-Mn vs. J-Mn). a, citrate cycle pathway (TCA cycle); b, snare interactions in vesicular transport. C-CK, control of Combier; C-Mn, Mn treatment of Combier; J-CK, control of Jinshou; J-Mn, Mn treatment of Jinshou. (XLS 142 kb)
Fig. S5
Schematic diagram of the transcript changes of plant roots in the KEGG pathway between two grapevine cultivars under Mn-treated or control conditions (C-CK vs. C-Mn; J-CK vs. J-Mn). a, glycolysis metabolism; b, photosynthesis; c, glycerophospholipid metabolism; d, N-glycan biosynthesis; e, glyoxylate and dicarboxylate metabolism; f, pyruvate metabolism; g, steroid biosynthesis; h, brassinosteroid biosynthesis. C-CK, control of Combier; C-Mn, Mn treatment of Combier; J-CK, control of Jinshou; J-Mn, Mn treatment of Jinshou. (XLS 466 kb)
Table S1
Primer sequences of the candidate genes fo qRT-PCR. (XLS 21 kb)
Table S2
Significantly enriched gene ontology (GO) terms in the Mn/CK root libraries of the two grape cultivars. (XLS 32 kb)
Table S3
Significant pathway enrichment analysis of the KEGG pathway in each pair of grape root libraries. (XLS 37 kb)
Table S4
DEGs for DNA repair in the roots between Combier and Jinshou. (XLS 20 kb)
Table S5
DEGs for auxin synthesis or oxidation in the roots between Combier and Jinshou. (XLS 22 kb)
Table S6
DEGs for stress-related protein in the roots between Combier and Jinshou. (XLS 62 kb)
Rights and permissions
About this article
Cite this article
Yao, Y., Xiao, X., Ou, Y. et al. Root transcriptome analysis on the grape genotypes with contrast translocation pattern of excess manganese from root to shoot. Plant Soil 387, 49–67 (2015). https://doi.org/10.1007/s11104-014-2279-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2279-2