Abstract
Background and aims
: The factors controlling litter decomposition and soil microbial community are important regulators of biogeochemical processes. Here we aim to explore controls on litter decomposition dynamics and soil microbial community composition in temperate forest by comparing three Korean pine forests along an altitudinal gradient.
Methods
: Single- and mixed-species litter decomposition rates were determined by the litterbag method and soil microbial community composition was characterized by PLFAs.
Results
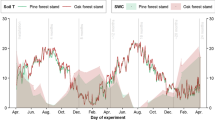
: Litter decomposition rates decreased with altitude regardless of litter type, and were controlled by temperature. Generally, fine root litter decomposed more rapidly than leaf litter, and mixed-species litter decomposed faster than single-species. Soil microbial biomass C and N decreased with altitude and varied differently among forest types in response to soil temperature and nutrient status. Fungal: bacterial PLFA ratios were significantly larger in forests receiving relatively poor litter quality inputs. Soil temperature, soil water content, total soil N and P were all directly related to the changes in total PLFAs among three forests.
Conclusions
: In these Korean pines dominated temperate forests, environmental changes associated with altitude gradient drive decomposition dynamics and soil microbial community composition. This implies that climate change might be an important factor affecting these systems in the future.





Similar content being viewed by others
References
Abiven S, Recous S, Reyes V, Oliver R (2005) Mineralisation of C and N from root, stem and leaf residues in soil and role of their biochemical quality. Biol Fertil Soils 42:119–128
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Bardgett RD, Kandeler E, Tscherko D, Hobbs PJ, Bezemer TM, Jones TH, Thompson LJ (1999) Below-ground microbial community development in a high temperature world. Oikos 85:193–203
Bauhus J, Barthel R (1995) Mechanisms for carbon and nutrient release and retention in beech forest gaps. Plant Soil 168:585–592
Berg B, Berg MP, Bottner P, Box E, Breymeyer A, de Anta RC, Couteaux M, Escudero A, Gallardo A, Kratz W (1993) Litter mass loss rates in pine forests of Europe and Eastern United States: some relationships with climate and litter quality. Biogeochemistry 20:127–159
Bloomfield J, Vogt KA, Vogt DJ (1993) Decay rate and substrate quality of fine roots and foliage of two tropical tree species in the Luquillo Experimental Forest, Puerto Rico. Plant Soil 150:233–245
Bossio D, Scow K (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bray SR, Kitajima K, Mack MC (2012) Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol Biochem 49:30–37
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Global Chang Biol 15:1339–1355
Diaz-Ravina M, Acea M, Carballas T (1995) Seasonal changes in microbial biomass and nutrient flush in forest soils. Biol Fertil Soils 19:220–226
Eiland F, Klamer M, Lind AM, Leth M, Bååth E (2001) Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb Ecol 41:272–280
Fang J, Chen A, Peng C, Zhao S, Ci L (2001) Changes in forest biomass carbon storage in China between 1949 and 1998. Science 292:2320–2322
FAO-UNESCO (1988) Soil Map of the World, Revised Legend. World Soil Resource Rep 60, FAO, Rome
Feng X, Simpson MJ (2009) Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol Biochem 41:804–812
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Frey SD, Drijber R, Smith H, Melillo J (2008) Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem 40:2904–2907
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Gallardo A, Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol Biochem 26:1409–1415
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513
Hogberg MN, Hogberg P, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150:590–601
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J stat 6:65–70
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sc 165:382–396
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambrige, pp 224–235
Lovell RD, Jarvis SC, Bardgett RD (1995) Soil microbial biomass and activity in long-term grassland: effects of management changes. Soil Biol Biochem 27:969–975
Majdi H (2004) Root and needle litter decomposition responses to enhanced supplies of N and S in a Norway spruce forest in southwest Sweden. Plant Biosyst 138:225–230
Marschner P, Umar S, Baumann K (2011) The microbial community composition changes rapidly in the early stages of decomposition of wheat residue. Soil Biol Biochem 43:445–451
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Norris MD, Avis PG, Reich PB, Hobbie SE (2013) Positive feedbacks between decomposition and soil nitrogen availability along fertility gradients. Plant Soil 367:347–361
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Ostertag R, Hobbie SE (1999) Early stages of root and leaf decomposition in Hawaiian forests: effects of nutrient availability. Oecologia 121:564–573
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Rantalainen ML, Kontiola L, Haimi J, Fritze H, Setälä H (2004) Influence of resource quality on the composition of soil decomposer community in fragmented and continuous habitat. Soil Biol Biochem 36:1983–1996
Rinnan R, Michelsen A, Bååth E, Jonasson S (2007) Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Global Chang Biol 13:28–39
Salamanca EF, Kaneko N, Katagiri S (1998) Effects of leaf litter mixtures on the decomposition of Quercus serrata and Pinus densiflora using field and laboratory microcosm methods. Ecol Eng 10:53–73
Salinas N, Malhi Y, Meir P, Silman M, Roman CR, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M, Farfan F (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977
Schimel JP, Clein JS (1996) Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biol Biochem 28:1061–1066
Schindlbacher A, Rodler A, Kuffner M, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S (2011) Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol Biochem 43:1417–1425
Scowcroft P, Turner DR, Vitousek PM (2000) Decomposition of Metrosideros polymorpha leaf litter along elevational gradients in Hawaii. Global Chang Biol 6:73–85
Shaver GR, Canadell J, Chapin F III, Gurevitch J, Harte J, Henry G, Ineson P, Jonasson S, Melillo J, Pitelka L (2000) Global Warming and Terrestrial Ecosystems: A Conceptual Framework for Analysis: Ecosystem responses to global warming will be complex and varied. Ecosystem warming experiments hold great potential for providing insights on ways terrestrial ecosystems will respond to upcoming decades of climate change. Documentation of initial conditions provides the context for understanding and predicting ecosystem responses. Bioscience 50:871–882
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 93:345–354
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Ushio M, Wagai R, Balser TC, Kitayama K (2008) Variations in the soil microbial community composition of a tropical montane forest ecosystem: Does tree species matter? Soil Biol Biochem 40:2699–2702
van der Heijden MG, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Vance ED, Brookes P, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Von Lützow M, Zelles L, Scheunert I, Ottow J (1992) Seasonal effects of liming, irrigation, and acid precipitation on microbial biomass N in a spruce (Picea abies L.). for soil Biol Fertil Soils 13:130–134
Vos VC, van Ruijven J, Berg MP, Peeters ET, Berendse F (2013) Leaf litter quality drives litter mixing effects through complementary resource use among detritivores. Oecologia 173:269–280
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–284
Wang QK, Wang SL (2007) Soil organic matter under different forest types in Southern China. Geoderma 142:349–356
Wang H, Liu S, Mo J (2010a) Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 335:289–298
Wang S, Ruan H, Han Y (2010b) Effects of microclimate, litter type, and mesh size on leaf litter decomposition along an elevation gradient in the Wuyi Mountains, China. Ecol Res 25:1113–1120
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol Biochem 30:1627–1637
Xiao CW, Janssens IA, Sang WG, Wang RZ, Xie ZQ, Pei ZQ, Yi Y (2010) Belowground carbon pools and dynamics in China’s warm teperate and sub-tropical deciduous forests. Biogeosciences 7:275–287
Yang H, Li D (1985) Distribution patterns of dominant tree species on northern slope of Changbai Mountain. Res Forest Ecosyst 5:1–4 (In chinese)
Yang K, Zhu J, Zhang M, Yan Q, Sun OJ (2010) Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J. Plant Ecol 3:175–182
Zhong Z, Makeschin F (2006) Differences of soil microbial biomass and nitrogen transformation under two forest types in central Germany. Plant Soil 283:287–297
Zhou Y, Su J, Janssens IA, Zhou G, Xiao C (2014) Fine root and litterfall dynamics of three Korean pine (Pinus koraiensis) forests along an altitudinal gradient. Plant Soil 374:19–32
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (No. 31370462), and State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, the Chinese Academy of Sciences. We thank Guanhua Dai (the Research Station of Changbai Mountain Forest Ecosystem, Chinese Academy of Sciences) for his assistance with field work. We further thank Professor Thomas W. Boutton and Dr. Carissa Wonkka (Texas A&M University) for their helpful comments on this manuscript. Additional thanks are extended to two anonymous reviewers, whose suggestions helped to improve this manuscript. We also gratefully acknowledge the Research Station of Changbai Mountain Forest Ecosystem of Chinese Academy of Sciences for help with logistics, and the Changbai Mountain Nature Reserve for permission to access the study sites.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Zhou, Y., Clark, M., Su, J. et al. Litter decomposition and soil microbial community composition in three Korean pine (Pinus koraiensis) forests along an altitudinal gradient. Plant Soil 386, 171–183 (2015). https://doi.org/10.1007/s11104-014-2254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2254-y