Abstract
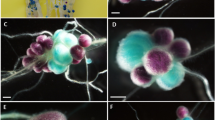
The stages in the nodulation process that determined the competitiveness of R. leguminosarum bv. trifolii (Rlt) strain 20–15, which proved to be highly competitive for nodulation in Iceland fields tests over several years, is analysed. White clover (Trifolium repens L.) roots were inoculated with inoculum mixtures containing three strains (Rlt 20-15, Rlt 8-9 and Rlt 32-28) in different proportions and cell densities. Competitiveness in root colonization, formation of infection threads and nodule development was assessed for Rlt 20-15 and its weakest competitor, Rlt 32-28. ERIC-polymerase chain reaction (PCR) DNA fingerprinting was used to identify inoculated strains recovered from root surfaces and individual nodules. GFP or DsRed tagged strains were used to determine identity in root hairs and nodules. Both strains colonized the root equally at all inoculum ratios tested. But, Rlt 20-15 initiated significantly more infection threads and formed more nodules than Rlt 32-28. These results show that Rlt 20-15 expresses its nodulation competitiveness during infection, either at infection thread initiation or during successive growth in the infection threads. The data presented support earlier observations that this strain competed well in the field in spite of its inferior ability to survive in the soil.




Similar content being viewed by others
References
Agresti A (2002) Categorical Data Analysis, 2nd edn. Wiley, New Jersey
Bhuvaneswari TV, Bhagwat AA, Bauer WD (1981) Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol 68:1144–1149
Brockwell J, Bottomley PJ, Thies JE (1995) Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. Plant Soil 174:143–180. doi:10.1007/BF00032245
Brockwell J, Gault RR, Morthorpe LJ, Peoples MB, Turner GL, Bergersen FJ (1989) Effects of soil nitrogen status and rate of inoculation on the establishment of populations of Bradyrhizobium japonicum and on the nodulation of soybeans. Aust J Agric Res 40:753–762
Broughton WJ, Dilworth MJ (1970) Plant nutrient solutions: In Somasegaran P, Hoben HJ (eds) Methods in Legume-Rhizobium Technology-Handbook for rhizobia Niftal Project, Univ. of Hawaii. pp 245–249
de Bruijn FJ (1992) Use of REP (Repetitive Extragenic Palindromic and Enterobacterial Repetitive Intergenic Consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol 58:2180–2187
Dowling DN, Broughton WJ (1986) Competition for nodulation of legumes. Annu Rev Microbiol 40:131–157. doi:10.1146/annurev.mi.40.100186.001023
Duodu S, Bhuvaneswari TV, Gudmundsson J, Svenning MM (2005) Symbiotic and saprophytic survival of three unmarked Rhizobium leguminosarum biovar trifolii strains introduced into the field. Environ Microbiol 7:1049–1058. doi:10.1111/j.1462-2920.2005.00789.x
Duodu S, Carlsson G, Huss-Danell K, Svenning MM (2007) Large genotypic variation but small variation in N2 fixation among rhizobia nodulating red clover in soils of northern Scandinavia. J Appl Microbiol 102:1625–1635. doi:10.1111/j.1365-2672.2006.03196.x
Gage DJ (2002) Analysis of infection thread development using gfp-and DsRed- expressing Sinorhizobium meliloti. J Bacteriol 184:7042–7046. doi:10.1128/JB.184.24.7042-7046.2002
Herridge DF, Roughley RJ, Brockwell J (1984) Effect of rhizobia and soil nitrate on the establishment and functioning of the soybean symbiosis in the field. Aust J Agric Res 35:149–161. doi:10.1071/AR9840149
Herouart D, Baudouin E, Frendo P, Harrison J, Santos R, Jamet A, Van de Sype G, Touati D, Puppo A (2002) Reactive oxygen species, nitric oxide and glutathione: a key role in the establishment of the legume: Rhizobium symbiosis. Plant Physiol Biochem 40:619–624. doi:10.1016/S0981-9428(02)01415-8
Jensen JB, Ampomah OY, Darrah R, Peters NK, Bhuvaneswari TV (2005) Role of trehalose transport and utilization in Sinorhizobium meliloti-alfalfa interactions. Mol Plant Microbe Interact 18:694–702. doi:10.1094/MPMI-18-0694
Kirwan LA, Lüscher MT, Sebastià JA et al (2007) Evenness drives consistent diversity effects in intensive grassland systems across 28 European sites. J Ecol 95:530–539. doi:10.1111/j.1365-2745.2007.01225.x
Leung K, Yap K, Dashti N, Bottomley PJ (1994) Serological and ecological characteristics of a nodule-dominant serotype from an indigenous soil population of Rhizobium leguminosarum bv. trifolii. Appl Environ Microbiol 60:408–415
Ma W, Charles TC, Glick BR (2004) Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Sinorhizobium meliloti increases its ability to nodule alfalfa. Appl Environ Microbiol 70:5891–5897. doi:10.1128/AEM.70.10.5891-5897.2004
Merrick MJ, Edwards RA (1995) Nitrogen control in bacteria. Microbiol Rev 59:604–622
Okazaki S, Nukui N, Sugawara M, Minamisawa K (2004) Rhizobial strategies to enhance symbiotic interactions: Rhizobitoxine and 1-aminocyclopropane-1-carboxylate deaminase. Microbes Environ 19:99–111. doi:10.1264/jsme2.19.99
Sessitsch A, Howieson JG, Perret X, Antoun H, Martínez-Romero E (2002) Advances in Rhizobium Research. Crit Rev Plant Sci 21:323–378. doi:10.1080/0735-260291044278
Somasegaran P, Hoben HJ (1994) Handbook for Rhizobia: Methods in Legume-Rhizobium Technology. Springer-Verlag, New York, pp 366–369
Svenning MM, Gudmundsson J, Fagerli I-L, Leinonen P (2001) Competition for nodule occupancy between introduced strains of Rhizobium leguminosarum biovar trifolii and its influence on plant production. Ann Bot (Lond) 88:781–787. doi:10.1006/anbo.2001.1484
Triplett EW, Sadowsky MJ (1992) Genetics of competition for nodulation of legumes. Annu Rev Microbiol 42:399–428. doi:10.1146/annurev.mi.46.100192.002151
Young RR, Brockwell J (1992) Influence of soil pH on the development of symbiosis in field-grown acid-sensitive and acid-tolerant annual medics. Aust J Exp Agric 32:167–173. doi:10.1071/EA9920167
Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6923–6831. doi:10.1093/nar/19.24.6823
Vlassak KM, Vanderleyden J (1997) Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci 16:163–219. doi:10.1080/713608146
Wardlaw AC (2000) Practical statistics for experimental biologists, Second edition. John Wiley & Sons, Ltd., New York, N. Y
Zaat SAJ, van Brussel AAN, Tak T, Lugtenberg BJJ, Kijne JW (1989) The ethylene-inhibitor aminoethoxyvinylglycine restores normal nodulation by Rhizobium leguminosarum biovar viciae on Vicia sativa subsp. nigra by suppressing the ‘Thick and short roots’ phenotype. Planta 177:141–150. doi:10.1007/BF00392802
Acknowledgements
This work was supported by the Norwegian Research Council grant (126312/110), as part of NKJ (Nordic Joint Committee for Agricultural Research) project 106- Exploring legume-Rhizobium symbioses for sustainable agriculture. Travel support to Ireland for the statistical analysis was funded by COST 852 short-term scientific missions. We thank Dr. Daniel J. Gage at the University of Connecticut, USA, for kindly providing us with the GFP and DsRed plasmids. Coby Weber is thanked for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Harsh P. Bais.
Rights and permissions
About this article
Cite this article
Duodu, S., Brophy, C., Connolly, J. et al. Competitiveness of a native Rhizobium leguminosarum biovar trifolii strain for nodule occupancy is manifested during infection. Plant Soil 318, 117–126 (2009). https://doi.org/10.1007/s11104-008-9822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9822-y