Abstract
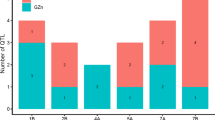
Zinc (Zn) deficiency is a widespread problem which reduces yield and grain nutritive value in many cereal growing regions of the world. While there is considerable genetic variation in tolerance to Zn deficiency (also known as Zn efficiency), phenotypic selection is difficult and would benefit from the development of molecular markers. A doubled haploid population derived from a cross between the Zn inefficient genotype RAC875-2 and the moderately efficient genotype Cascades was screened in three experiments to identify QTL linked to growth under low Zn and with the concentrations of Zn and iron (Fe) in leaf tissue and in the grain. Two experiments were conducted under controlled conditions while the third examined the response to Zn in the field. QTL were identified using an improved method of analysis, whole genome average interval mapping. Shoot biomass and shoot Zn and Fe concentrations showed significant negative correlations, while there were significant genetic correlations between grain Zn and Fe concentrations. Shoot biomass, tissue and grain Zn concentrations were controlled by a number of genes, many with a minor effect. Depending on the traits and the site, the QTL accounted for 12–81% of the genetic variation. Most of the QTL linked to seedling growth under Zn deficiency and to Zn and Fe concentrations were associated with height genes with greater seedling biomass associated with lower Zn and Fe concentrations. Four QTL for grain Zn concentration and a single QTL for grain Fe concentration were also identified. A cluster of adjacent QTL related to the severity of symptoms of Zn deficiency, shoot Zn concentration and kernel weight was found on chromosome 4A and a cluster of QTL associated with shoot and grain Fe concentrations and kernel weight was found on chromosome 3D. These two regions appear promising areas for further work to develop markers for enhanced growth under low Zn and for Zn and Fe uptake. Although there was no significant difference between the parents, the grain Zn concentration ranged from 29 to 43 mg kg−1 within the population and four QTL associated with grain Zn concentration were identified. These were located on chromosomes 3D, 4B, 6B and 7A and they described 92% of the genetic variation. Each QTL had a relatively small effect on grain Zn concentration but combining the four high Zn alleles increased the grain Zn by 23%. While this illustrates the potential for pyramiding genes to improve grain Zn, breeding for increased grain Zn concentration requires identification of individual QTL with large effects, which in turn requires construction and testing of new mapping populations in the future.

Similar content being viewed by others
References
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S et al (2006) Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Barker SJ, Stummer B, Gao L, Dsipain I, O, Conner PJ, Smith SE (1998) A mutant in lycopersicon esculentum Mill. with highly reduced VA mycorrhizal colonisation: isolation and preliminary characterization. Plant J 15:791–797
Broman KW, Hao Wu and with ideas from Gary Churchill and Saunak Sen and contributions from Brian Yandell (2006) qtl: Tools for analyzing QTL experiments. R package version 1.05-2
Butler DG, Cullis BR, Gilmour AR, Gogel BJ (2007) ASReml-R reference manual. Release 2.0. Technical report, Queensland Department of Primary Industries
Cakmak I (2000) Role of zinc in protecting plant cells from reactive oxygen species. New Phytol 146:185–205
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
Cakmak I, Atli M, Kaya R, Evliya H, Marschner H (1995) Association of high light and zinc deficiency in cold-induced leaf chlorosis in grapefruit and mandarin trees. J Plant Physiol 146:355–360
Cakmak I, Yılmaz A, Ekiz H, Torun B, Erenoglu B, Braun HJ (1996) Zinc deficiency as a critical problem in wheat production in Central Anatolia. Plant Soil 180:165–172
Cakmak I, Derici R, Torun B, Tolay I, Braun HJ, Schlegel R (1997) Role of rye chromosomes in improvement of zinc efficiency in wheat and triticale. Plant Soil 196:249–253
Cakmak I, Tolay I, Ozdemir A, Ozkan H, Kling CI (1999) Differences in zinc efficiency among and within diploid, tetraploid and hexaploid wheats. Ann Bot (Lond) 84:163–171
Cakmak I, Torun B, Ozturk L, Marschner H, Kalayci M (1998) Morphological and physiological differences in the response of cereals to zinc deficiency. Euphytica 100:349–357
Cakmak I, Torun A, Millet E, Feldman M, Fahima T, Korol A et al (2004) Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci Plant Nutr 50:1047–1054
Coleman RK, Gill GS, Rebetzke GJ (2001) Identification of quantitative trait loci for traits conferring weed competitiveness in wheat (Triticum aestivum L.). Aust J Agric Res 52:1235–1246
Distelfeld A, Cakmak I, Peleg Z, Ozturk L, Yazici AM, Budak H et al (2007) Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol Plant 129:635–643
Ellis MH, Spielmeyer W, Gale KR, Rebetzke GJ, Richards RA (2002) Perfect markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor Appl Genet 105:1038–1042
Fahima T, Distelfeld A, Peleg Z, Ozturk L, Yazici AM, Saranga Y et al (2006) Multiple QTL-effects on grain zinc, iron and protein concentrations localized within a 250-kb interval on chromosome 6BS of wheat. 8th International Congress of Plant Molecular Biology, 20–25 August 2006, Adelaide, Australia
Gao X, Zou C, Zhang SETM, Van Der Zee F, Hoffland E (2005) Tolerance to zinc deficiency in rice correlates zinc uptake and translocation. Plant Soil 278:253–261
Genc Y, McDonald GK, Graham RD (2000) Effect of seed zinc content on early growth of barley (Hordeum vulgare L.) under low and adequate soil zinc supply. Aust J Agric Res 51:37–46
Genc Y, McDonald GK, Graham RD (2002) A soil-based method to screen for zinc efficiency in seedlings and its ability to predict yield responses to zinc. Aust J Agric Res 53:409–421
Genc Y, Shepherd KW, McDonald GK, Graham RD (2003) Inheritance of tolerance to zinc deficiency in barley. Plant Breed 122:283–284
Genc Y, McDonald GK, Graham RD (2006) Contribution of different mechanisms to zinc efficiency in bread wheat during early vegetative stage. Plant Soil 281:353–367
Gilmour AR, Cullis BR, Gogel BJ, Thompson R (2006) ASReml Users Guide. Release 2.0. VSN International Ltd
Graham RD (1984) Breeding for nutritional characteristics in cereals. Adv Plant Nutr 1:57–102
Graham RD, Stangoulis JCR (2003) Trace element uptake and distribution in plants. J Nutr 133:1502S–1505S
Graham RD, Ascher JS, Hynes SC (1992) Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil 146:241–250
Graham RD, Senadhira D, Beebe S, Iglesias C, Monasterio I (1999) Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crops Res 60:57–80
Gregorio GB, Senadhira D, Mendoza RD, Manigbas NL, Roxas JP, Guerta CQ (2002) Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res 76:91–101
Guidet F, Rogowsky P, Taylor C, Song W, Langridge P (1991) Cloning and characterisation of a new rye-specific repeated sequence. Genome 34:81–87
Hacisalihoglu G, Hart JJ, Wang YH, Cakmak I, Kochian LV (2003) Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in wheat. Plant Physiol 131:595–602
Hackett CA, Meyer RC, Thomas WBT (2001) Multi-trait QTL mapping in barley using multivariate regression. Genet Res 77:95–106
Jansen RC (1994) Controlling the type i and type ii errors in mapping quantitative trait loci. Genetics 138:871–881
Lander ES, Green P (1987) Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci USA 84:2363–2367
Lonergan PF (2001) Genetic characterization of QTL mapping of zinc nutrition in barley (Hordeum vulgare L.). University of Adelaide, South Australia (PhD thesis)
Manly KF, Cudmore RH Jr, Meer JM (2001) Map Manager QTX, crossplatform software for genetic mapping. Mamm Genome 12:930–932
McDonald GK, Genc Y, Graham RD (2008) A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant Soil 306:49–55
Ozkan H, Brandolini A, Torun A, Altintas S, Eker S, Kilian B et al (2006) Natural variation and identification of microelements content in seeds of Einkorn Wheat (Triticum monococcum). In Proceedings of the 7th International Wheat Conference, 27 November–2 December 2005, Mar del Plata, Argentina. pp 455–462
Peck AW, McDonald GK, Graham GK (2008) Zinc nutrition influences the protein composition of flour in bread wheat (Triticum aestivum L.). J Cereal Sci 47:266–274
R Development Core Team (2006) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0
Rebetzke GJ, Appels R, Morrison AD, Richards RA, McDonald G, Ellis MH et al (2001) Quantitative trait loci on chromosome 4B for coleoptile length and early vigour in wheat (Triticum aestivum L.). Aust J Agric Res 52:1221–1234
Rengel Z (1999) Physiological mechanisms underlying differential nutrient efficiency of crop genotypes. In Mineral Nutrition of Crops-Fundamental Mechanisms and Implications. Food Products Press, New York, pp 227–265
Rengel Z, Graham RD (1995) Importance of seed Zn content for growth on Zn-deficient soil. I. Vegetative growth. Plant Soil 173:259–266
Rengel Z, Batten GD, Crowley DE (1999) Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res 60:27–40
Reuter DJ, Robinson JB (1997) Plant analysis. An interpretation manual, 2nd Edn. CSIRO, Australia
Rogowsky P, Guidet FLY, Langridge P, Shepherd KW, Koebner RMD (1991) Isolation and characterization of wheat–rye recombinants involving chromosome arm 1DS of wheat. Theor Appl Genet 82:537–544
Shi R, Li H, Tong Y, Jing R, Zhang F, Zou C (2007) Identification of quantitative trait locus of zinc and phosphorus density in wheat (Triticum aestivum L.) grain. Plant Soil 306:95–104
Smith A, Cullis BR, Thompson R (2005) The analysis of crop cultivar breeding and evaluation trials: an overview of current mixed model approaches. J Agric Sci 143:449–462
Tinker NA, Mather DE (1995) Methods for QTL analysis with progeny replicated in multiple environments. J Agric Genomics 1: http://wheat.pw.usda.gov/jag/
Vargas M, Van Eewijk FA, Crossa J, Ribaut JM (2006) Mapping QTLs and QTL × environment interaction for CIMMYT maize drought stress program using factorial regression and partial least squares methods. Theor Appl Genet 112:1009–1023
Verbyla AP, Eckermann PJ, Thompson R, Cullis BR (2003) The analysis of quantitative trait loci in multi-environment trials using a multiplicative mixed model. Aust J Agric Res 54:1395–1408
Verbyla AP, Cullis BR, Thompson R (2007) The analysis of QTL by simultaneous use of the full linkage map. Theor Appl Genet 116:95–111
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Williams KJ, Dennis JI, Smyl C, Wallwork H (2002) The application of species-specific assays based on the polymerase chain reaction to analyse Fusarium crown rot of durum wheat. Australas Plant Pathol 31:119–127
Wissuwa M, Ismail AM, Yanagihara S (2006) Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol 142:731–741
Wu G, Wilen RW, Robertson AJ, Gusta LV (1999) Isolation, chromosomal localization, and differential expression of mitochondrial manganese superoxide dismutase and chloroplastic copper/zinc superoxide dismutase genes in wheat. Plant Physiol 120:513–520
Yılmaz A, Ekiz H, Gültekin I, Torun B, Barut H, Karanlık S et al (1998) Effect of seed zinc content on grain yield and zinc concentration of wheat grown in zinc-deficient calcareous soils. J Plant Nutr 21:2257–2264
Zarcinas BA, Cartwright, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Acknowledgments
We wish to thank Mrs. Teresa Fowles and Mr. Lyndon Palmer for their help with ICP analyses and Mr. Jim Lewis for his assistance with the field trial. We greatly appreciate constructive comments from the editor and anonymous reviewers. This work was supported by Molecular Plant Breeding Cooperative Research Centre and The University of Adelaide, Australia. APV acknowledges the financial support by the Australian Grains Research and Development Corporation through Key Program 3 of the National Statistics Programme (DAN00085).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Responsibility: F. Zhao.
Appendix
Appendix
Statistical models: baseline models without QTL
The mixed model forms the basis of the analysis and is given by
where X, Z and Z l , and are known design matrices for the fixed effects, random effects and genetic effects respectively, τ is the vector of fixed effect parameters, u is a vector of random effects and e is a vector of residual random effects. These latter two effects are assumed independent, mean zero with covariance matrices G u and R and respectively. The form of G u and R will depend on the application.
We are specifically interested in the genetic line effects g. Two classes of analysis are required, firstly for the Zn scores and grain Zn and Fe concentrations, and secondly for the seedling traits. The two situations involve different models for g.
Zinc score
Since only the nil Zinc plants were scored, g represents a single set of genotypic line effects under the nil treatment, and it is assumed
where \(\sigma _g^2 \) is the genetic line variance and n g is the number of lines. The base model fitted was
where the bold terms are random effects. The term Type is a factor of three levels (DH, RAC875-2, Cascades) and hence distinguishes the DH lines from the parents. The Variety term represents the polygenic line effects.
It was assumed that the Error term was such that \(e \sim N\left( {0,\sigma ^2 {\text{I}}_{\text{n}} } \right)\), so that R = σ 2 I n . Thus is the genetic variance associated with the doubled haploid lines, while σ 2 is the residual or error variance.
Plant traits
Shoot dry matter, shoot Zn and Fe concentration were measured under both the Nil and the Zinc treatments, and in Experiments 1 and 2. The resulting four combinations will be considered a (structured) set of four environments. Thus we assume
where G is a 4 × 4 variance-covariance matrix that provides the genetic variances across lines for the trait grown under the two treatments in the two experiments (hence 4) and the genetic covariances of the trait between pairs of environments; G may be an unstructured (and hence fully parameterized) variance–covariance matrix, or it may take on another form. In the analysis of multi-environment trials, this matrix is taken to be a factor analytic (FA) structure (Smith et al. 2005). For the case of t environments, the FA model is given by
where if there are n f factors, f represents a vector of latent unobserved factors of size \(n_f n_g \times 1\), \( \wedge \) is t × n f an matrix of factor loadings for each of the treatments and each factor, and ξ is a residual random vector for genetic variation not explained by the factors. To ensure identifiability, \({\mathbf{f}} \sim {\text{N}}{\left( {{\mathbf{0}}{\text{,}}{\mathbf{I}}_{{n_{f} n_{g} }} } \right)}\) and \(\xi \sim N\left( {0,\Psi \otimes {\text{I}}_{n_g } } \right)\) where Ψ where is a diagonal matrix. These FA models provide a parsimonious structure in many applications (see Smith et al. 2005).
Dry matter was multiplied by 100 to improve the scale for both estimation and reporting. However, there was clear variance heterogeneity for this and the other traits and the analysis was conducted on the log-scale. For the three plant traits (seedling biomass, shoot Zn and Fe concentrations), the base model fitted was:
where Site represents the two experiments (Adelaide and Adana), Env is a factor of four levels, being a 2 × 2 factorial structure of level of Zinc (Nil, Zinc) by experiment (Adelaide, Adana) and Block reflects the randomized complete block design. The term Env.Variety allows for the interaction of genetic effects with the environment, and the latter is determined by the treatment by experiment combinations. The term at(Site).Block allows for separate block effects at each Site.
The Error term allowed for differing residual variances for each experiment by treatment combination; thus four residual variances were estimated.
Grain traits
Kernel weight, Zn and Fe concentration were measured on grain from the Zn-treated plants in Experiment 3 (the field trial). The model for g as given by (2) is again appropriate. The base model fitted for kernel weight was
where Block reflects the design of the field trial, and the random Column effect allows for between column variation in the field. The Error term allowed for spatial variation in the field as indexed by the row and column position of the plots (see Gilmour et al. 2006).
For grain Zn concentration, the base model fitted is given symbolically as
where terms are very similar to the kernel weight model. For grain Fe concentration, the base model fitted was:
where additional terms were required to account for smooth spatial field dependence across rows that was evident in the analysis.
Statistical Models for QTL analysis
Zinc score and grain traits
The approach used for QTL analysis is based on Verbyla et al. (2007) who provide an approach that uses the full linkage map simultaneously in a staged analysis. A working model is used in which all intervals are assumed to possibly contain a QTL. The vector of the sizes of QTL effects for all intervals is denoted by a and is assumed to be a random effect with mean zero and variance\(\left( {\sigma _a^2 } \right)\). If this variance is significant, an outlier detection technique is used to sequentially select putative QTL. At each selection step, the putative QTL interval is moved to the fixed effects part of the model and selection ceases when the between interval variation\(\left( {\sigma _a^2 } \right)\) is no longer statistically significant.
The initial mixed model that is used for the QTL analysis is given by
where the matrix M E consists of pseudo-markers that relate to intervals on the linkage map (see Verbyla et al. 2007 for details). An important summary of the contribution of the selected QTL is the percentage of genetic variance explained by the QTL. An overall measure is given by
where \(\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\frown}$}}{\sigma } ^{2}_{{g,b}}\) is the estimated polygenic genetic variance for the baseline model (that is, without marker effects) and \(\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\frown}$}}{\sigma } ^{2}_{{g,b}}\) is the equivalent estimated polygenic genetic variance after finding the QTL. This quantity is reported in the results for each analysis.
Plant traits
A common approach for QTL analysis was used for plant traits and is based on the whole genome approach of Verbyla et al. (2007) in the case of multi-environment and multi-trait data (based on work in preparation by Verbyla and Cullis). A FA model (Smith et al. 2005) is used to allow detection of QTL that affect the trait in multiple environments or under multiple treatments or both. The initial mixed model for QTL analysis in this case is given by
where the term\(\left( { \wedge _a \otimes {\mathbf{M}}_E } \right){\mathbf{f}}_a \) allows for a common QTL effect across the 4 environments but with differential association in terms of size of effect, and\(\left( {{\mathbf{I}} \otimes {\mathbf{M}}_E } \right)\xi _a \) allows for environment specific QTL effects. The selection of QTL proceeds by choosing common effects until that term is no longer statistically significant. Subsequently, environment specific QTL are selected using the second term. Once this term is no longer statistically significant, the selection process is concluded.
Note that there are four polygenic “traits”, and hence four polygenic variances. Thus the calculations given in equation (3) can be conducted for each of the polygenic variances as given by the diagonal elements of G. Not only can the percentage of genetic variance be given for the combination of location and treatment, the variances and the correlations between the four combinations can be reported both before QTL and after QTL analysis thereby allowing the assessment of the impact of the QTL selected. Thus in the analysis of the plant traits the estimated G before and after QTL analysis is presented and discussed.
Rights and permissions
About this article
Cite this article
Genc, Y., Verbyla, A.P., Torun, A.A. et al. Quantitative trait loci analysis of zinc efficiency and grain zinc concentration in wheat using whole genome average interval mapping. Plant Soil 314, 49–66 (2009). https://doi.org/10.1007/s11104-008-9704-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9704-3