Abstract
Fine root turnover plays an important role in the cycling of carbon and nutrients in ecosystems. Not much is known about fine root dynamics in tropical montane rainforests, which are characterized by steep temperature gradients over short distances. We applied the minirhizotron technique in five forest stands along an elevational transect between 1,050 and 3,060 m above sea level in a South Ecuadorian montane rainforest in order to test the influence of climate and soil parameters on fine root turnover. Turnover of roots with diameter < 2.0 mm was significantly higher in the lowermost and the uppermost stand (0.9 cm cm−1 year−1) than in the three mid-elevation stands (0.6 cm cm−1 year−1). Root turnover of finest roots (d < 0.5 mm) was higher compared to the root cohort with d < 2.0 mm, and exceeded 1.0 cm cm−1 year−1 at the lower and upper elevations of the transect. We propose that the non linear altitudinal trend of fine root turnover originates from an overlapping of a temperature effect with other environmental gradients (e.g. adverse soil conditions) in the upper part of the transect and that the fast replacement of fine roots is used as an adaptive mechanism by trees to cope with limiting environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fine root turnover (i.e. the replacement of root mass within a year) represents one of the major carbon and nutrient sources in soils and thus plays an important role in ecosystem carbon cycling (Gill and Jackson 2000). Numerous studies on fine root dynamics in temperate forests are now available (e.g. Santantonio and Grace 1987; Aerts et al. 1989; Hendrick and Pregitzer 1992; Pregitzer et al. 1993; Hendrick and Pregitzer 1996; Joslin et al. 2000; Mainiero and Kazda 2006). In contrast, studies on fine root turnover in tropical forests are still scarce and were mostly conducted in lowland forests (e.g. Jordan and Escalante 1980; Cuevas and Medina 1988; Green et al. 2005; Silver et al. 2005; Trumbore et al. 2006). Even less is known about fine root dynamics in tropical montane forests despite their acknowledged importance in carbon cycling. Recent studies showed that high elevation tropical montane forests may possess a remarkably high fine root biomass due to a pronounced carbon allocation to the roots in the trees (Leuschner et al. 2007; Moser et al. 2008; Hertel and Leuschner 2008).
A global meta-analysis indicates that root turnover increases from boreal to tropical regions as a consequence of increased root maintenance respiration, a more rapid nutrient mineralization and a higher pathogen activity at higher temperatures (Gill and Jackson 2000). The effects of soil resource availability on fine root longevity are less clear than those of climatic factors, but might also be important variables explaining patterns of fine root dynamics (Pregitzer et al. 1993; Vogt et al. 1993; Pregitzer et al. 2000). Along a natural fertility gradient in Hawaii, root dynamics were related to nutrient availability, but N availability had a smaller effect than P availability (Ostertag 2001). In a northern hardwood forest, Burton et al. (2000) found root lifespan to be longer where N availability was higher. However, there are data both supporting (Alexander and Fairley 1983; Vogt et al. 1986; Pregitzer et al. 1993) and refuting (Roy and Singh 1995; Eissenstat and Yanai 1997; Nadelhoffer 2000; Yavitt and Wright 2001; Powers et al. 2005) the assumption that roots live longer in more fertile soils.
Nutrient mineralization in tropical montane forest is slowed down due to low temperatures and sometimes high soil water content (Tanner 1981; Vitousek and Sanford 1986; Benner et al. 2008). In their review, Benner et al. (2008) conclude that montane forests are usually nitrogen-limited and often also phosphorus-limited. Soethe et al. (2007) found decreasing fine root nutrient concentrations with increasing altitude, which is also evidence for nutrient limitation in tropical montane forests. Thus, not only low temperature but also nutrient limitation and, probably, other factors may affect fine root turnover in tropical forests at high altitudes.
We applied the minirhizotron technique in five south Ecuadorian montane rainforest stands along an altitudinal transect between 1,050 and 3,060 m above sea level (a.s.l.) in order to assess fine root dynamics by direct observation. Along this 2,000-m altitudinal transect we tested for temperature and soil effects on fine root production, mortality and turnover. We hypothesized that fine root turnover decreases with decreasing temperature and increasing altitude along the transect. It was further assumed that in the upper part of the altitudinal transect a temperature effect on root turnover is suppressed by adverse soil conditions, resulting in enhanced root turnover rates at the highest elevation.
Materials and methods
Study sites
The study was conducted on the Eastern slopes of the South Ecuadorian Andes in the provinces of Loja and Zamora-Chinchipe. Five forest stands at 1,050, 1,540, 1,890, 2,380, and 3,060 m a.s.l. were selected, representing an altitudinal gradient of 2,000 m. The two lowermost stands were located near the village of Bombuscaro in the area of the Podocarpus National Park. The stand at 1,050 m represents an upper pre-montane tropical moist forest with dominant tree species of the families Annonaceae, Mimosaceae, Moraceae, Myrtaceae and Sapotaceae. The stand at 1,540 m is a lower montane tropical moist forest with Arecaceae, Lauraceae, Melastomataceae and Rubiaceae being the most widespread families present (Homeier et al. 2008). The stands at 1,890 and 2,380 m were situated in the forest reserve of the Estación Científica San Francisco, and consist of mid-montane and upper-montane tropical moist forest, respectively. The most important tree species in terms of abundance in the stand at 1,890 m belong to the Euphorbiaceae, Lauraceae, Melastomataceae and Rubiaceae, and in the case of the stand at 2,380 m to the Aquifoliaceae, Clusiaceae, Cunoniaceae, Ericaceae and Rubiaceae (Homeier et al. 2008). The uppermost stand at 3060 m represents an elfin forest close to the tree line in the upper montane Cajanuma region of Podocarpus National Park; dominant trees belong to the Aquifoliaceae, Clusiaceae, Cunoniaceae, Ericaceae and Rubiaceae. Except for the stand at 1,540 m, all sites were situated on moderately steep slopes (26° to 31°) facing northeast to northwest (Table 1). The mean canopy height decreased from 32 m at the lowermost stand to 9 m at the uppermost stand, and mean tree height decreased accordingly from 15.6 to 5.2 m. Fine root biomass increased from 2.7 t ha−1 at 1,050 m to 10.8 t ha−1 at 3,060 m (Moser et al. 2008).
The climate of the region is influenced most of the year by easterly winds from the Amazonian lowlands transporting humid air masses to the slopes. Rainfall at 1,050, 1,540 and 1,890 m a.s.l. averages to about 2,000 mm year−1, whereas precipitation is ca. 5,000 mm year−1 at 2,380 m a.s.l. and 4,500 mm year−1 at 3,060 m a.s.l. (P. Emck and M. Richter, unpublished data; Moser et al. 2008). There is no marked dry season in the region, but lower rainfall during the months of October to January is common. The climate can be classified as humid to perhumid, with 11 to 12 humid months (Richter 2003). Air temperature and relative air humidity of the stands were measured 1.5 m above the forest floor with Hygroclip S temperature and humidity sensors (Rotronic AG, Switzerland). Annual mean air temperature decreased from 19.4°C at 1,050 m a.s.l. to 9.4°C at 3,060 m a.s.l., representing an average temperature lapse rate of 5 K km−1 along the slope (Table 1). Relative air humidity was high and increased slightly from 89% to 94% along the transect. The volumetric water content of the organic layer was continuously recorded with time domain reflectometry sensors, and increased from 29.7 vol.% at the lowermost stand to 49.1 vol.% at the uppermost stand (Table 1).
The soils of the forest stands derived from granodiorites at the two lowermost sites and from metamorphous shale and quartzite bedrock at the higher altitudes. Although the soils at all sites were acidic and relatively nutrient-poor (Schrumpf et al. 2001), the pH(CaCl2) values in the upper mineral soil (0–30 cm) decreased from 3.9 at 1,050 m to 2.9 at 3,060 m (S. Iost, unpublished data). A strong increase in the C/N ratio of the organic layer (from 22 to 63 g g−1) and in the depth of the organic layer thickness (from 48 to 435 mm) was found with increasing altitude (S. Iost, unpublished data).
Measurement of fine root growth and death
In each forest stand, a study plot of 20 × 20 m was selected in patches of undisturbed forest with a closed canopy that were representative for the forest community of the respective altitude. In each plot, ten transparent minirhizotron tubes with an external diameter (d) of 70 mm were installed in June 2005 at randomly selected locations. They were placed at a 90° angle to the slope and inserted to a depth of ca. 40 cm. In the lowermost stand with a very shallow soil, most of the tubes could not be inserted to a soil depth greater than 10 cm. Holes were dug with a soil corer that had the same diameter as the tubes, which ensured a tight contact of the tubes to the surrounding soil. To prevent entrance of water into the tubes, the bottom was sealed and the top end was covered with a removable cap. Those parts of the tubes that extended above the soil surface were covered with black tape to avoid incidence of light that might influence root growth. A circular plastic foil with a diameter of 16 cm was placed around the tubes on the soil surface to prevent water runoff on the surface of the tubes which could attract root growth.
For monitoring root growth, a root scanner system (CI-600 Root Growth Monitoring System, Fa. CID, USA) was used in the tubes from September 2005 to January 2007 at monthly intervals. Due to shortage in personnel we were not able to monitor root growth between April and July 2006. Five months after installation of the tubes we recognized a steady-state of visible root length in most of the tubes in all stands except for the uppermost stand. Hence, only data from November 2005 onwards were considered for analysis to avoid artifacts due to disturbance during tube installation (Hendrick and Pregitzer 1996; Majdi 1996; Joslin and Wolfe 1999). In each scanning procedure, we recorded a 345° sector of a soil compartment of 20 cm length at the tube’s inner surface (i.e. a total soil surface area of 219.9 cm2 per image). In this study, only the uppermost 10 cm below the soil surface were considered for analysis of fine root dynamics, as the very shallow soil at the lowermost site did not allow a deeper placement of the tubes. Earlier studies on fine root distribution in the same stands had shown a strong exponential decrease in fine root mass density with soil depth with the consequence that more than 50% of the fine root biomass of the profile total was located in the uppermost 10 cm of the soil (Röderstein et al. 2005; Moser et al. 2008). This justifies the focus on root growth analysis of the uppermost soil horizons.
The images were analyzed with the program WinRHIZO Tron (Règent, Quebec, Canada). Root length and diameter were traced manually on the screen for each image. For analyzing alteration in root length over time, the respective previous image was superposed over the current image. This allowed the identification of root sections originating from recent root growth or sections that had disappeared due to root death and decomposition. Software-assisted data analysis gave the length and surface area of recently born and disappeared root sections. Only fine roots (diameter <2 mm) were taken into account, which were grouped into two diameter classes (<2 mm and <0.5 mm) for further analysis.
By comparing visible and disappeared fine root length of current and previous images, cumulative root length production and root length loss were calculated per month (unit: cm month−1, Buckland et al. 1993; Majdi 1996). Relative root length production and relative root length loss were calculated by relating root length production or loss to the visible root length at the previous measuring date (cm cm−1 month−1). By extrapolating monthly relative root length loss to a full year (i.e. mean loss in a tube times 12), this data allowed for an estimation of average annual root loss rate of the visible fine root population in the rhizotron tubes (Nadelhoffer 2000). We equate annual root loss rate with “root turnover” by relating loss to the standing stock of fine root length in the image.
Statistical analysis
Based on ten replicate measurements (tubes) per stand, we calculated means and standard errors for all parameters. After applying a Shapiro–Wilk test to test for normality, a non-parametric global analysis of significant effects (Kruskal–Wallis test) and a Mann–Whitney two-sample test (U-test) were used to analyse significant differences in root length production, loss and turnover between the five stands. As we observed a significant variation of mean vapor pressure deficit (VPD) in the study period as the most obvious change in environmental conditions, we examined the relationship between mean VPD and root dynamics by means of a linear regression analysis (i.e. mean VPD 30 days prior to image recording was related to relative root length production and loss of the respective month). Spearman’s rank correlation was used to detect significant influences of environmental factors on root production parameters. All calculations were done with SAS/STAT software (p < 0.05).
Results
Fine root populations present
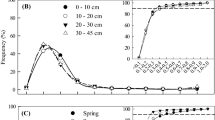
The highest mean root length visible in the minirhizotron tubes was recorded at the two mid-elevation stands at 1,540 m and 1,890 m (320 and 220 cm per image surface area of 219.9 cm2, respectively). The lowermost stand (1,050 m) and the two uppermost stands (2,380 and 3,060 m) showed a considerably smaller root length (Fig. 1). It became evident that steady state conditions of root turnover were reached earlier at the lower elevations. Except for the stand at 3,060 m root length did not increase significantly from December 2005 onwards. At the highest site root length was still increasing after 12 months of image recording (i.e. 18 months after tube installation). The average diameter of the fine root populations visible at the rhizotron tubes did not vary markedly among the stands: it varied between 0.55 and 0.68 mm and showed no significant elevational trend (Table 2). The percentage of fine roots with a diameter less than 0.5 mm ranged between 27% and 42%. While the percentage of these small roots was comparable in the two lowermost and the uppermost stand, the trees at 1,890 and 2,380 m tended to have a higher proportion of thicker roots.
Fine root production and loss
Monthly fine root length production showed no clear elevational trend: highest root length productivity was found in the two mid-elevation stands at 1,540 and 1,890 m (Fig. 2a), while root length production was lowest at 2,380 m. Both the lowermost and the uppermost stand showed medium root length production rates. Root length production of finest rootlets (d < 0.5 mm) was not significantly different among the five stands except for the stand at 2,380 m. Finest roots contributed by 30–50% to the total fine root (d < 2.0 mm) production. Hence root length production of the smallest roots was disproportionably high related to the standing root length stock. The proportion of finest root production was especially high at the lowermost and the uppermost stand.
Monthly root length loss also showed no clear elevational trend (Fig. 2b). As for root length production, monthly loss in root length was highest in the stand at 1,540 m. At 1,050 and 1,890 m monthly root length loss was much lower (about half). The lowest root length loss was recorded in the stand at 2,380 m. The uppermost stand at 3,060 m showed a significantly higher root length loss than the stand at 2,380 m. Roots <0.5 mm in diameter contributed to a large extent to length loss of roots <2.0 mm. However, monthly loss of finest roots was highest at the two lowermost stands, and lower in the mid- and high-elevation stands.
A different view arose when production and loss of fine roots were related to the root length present around the tubes at the beginning of a respective observation period. Except for the lowermost stand, relative root length production (RRLP) increased significantly with elevation (Fig. 3a). In the diameter class <2.0 mm lowest RRLP was found at 1,540 m (0.07 cm cm−1 month−1), while the uppermost stand showed highest RRLP (0.19 cm cm−1 month−1). The RRLP of fine roots in the lowermost stand was comparably high as in the stand at 2,380 m. Roots of diameters <0.5 mm showed higher RRLP than the root population with a diameter <2.0 mm. This was especially evident for the lowermost site, were RRLP of the small diameter class was as twice as high than of the larger diameter class. However, the increase in RRLP from 1,540 to 3,060 m was even more pronounced in the finest roots than in the fine roots (<2.0 mm).
Regarding monthly means of relative root length loss (RRLL; Fig. 3b) a similar elevational trend was observed as in relative root length production. In the diameter class <2.0 mm, the three mid-elevations stands showed RRLL of about 0.05 cm cm−1 month−1, whereas the lowermost and the uppermost stand had RRLL values of 0.08 cm cm−1 month−1. Except for the stand at 1,540 m, a higher RRLL was recorded in the small diameter class than in the larger diameter class, but the differences were less pronounced than with respect to RRLP. The highest relative root length loss with more than 0.1 cm cm−1 month−1 was recorded at the lowermost site. However, RRLL at the uppermost stand was nearly as high and differed not significantly from the lowermost site.
Fine root turnover
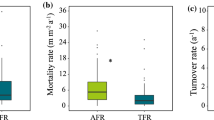
Roots in the lowermost and the two uppermost stands had a higher annual root turnover (i.e. loss per root standing stock) than the roots in the mid-elevation stands (1,540 and 1,890 m) (Fig. 4). Roots of diameters <0.5 mm had a considerably higher turnover than roots of <2.0 mm (Fig. 4). However, this difference was only significant in the stands at 1,050 and 2,380 m.
While highest turnover in the diameter class <2.0 mm occurred in the uppermost stand, the highest turnover of roots with diameters <0.5 mm was recorded in the lowermost stand (1.4 cm cm−1 year−1), followed by the two uppermost stands. Turnover of fine roots (<2.0 mm) was significantly higher at the lowermost and the uppermost stands (about 0.9 cm cm−1 year−1) compared to the mid-altitudes, where turnover was <0.6 cm cm−1 year−1. Annual root turnover of the <0.5 mm diameter roots showed a continuous increase with elevation from the 1,540 m to the 3,060 m stand.
Influence of environmental factors
A striking feature of the microclimate along the transect was that VPD inside the stands was much more variable over time at the lower elevations than higher upslope (Table 1). At the two lower-most stands a very high variability of mean VPD 30 days preceding the dates of images recording was found, which ranged between 1.5 and 8 hPa for the different observation periods. Variability in mean VPD at the higher altitudes was considerably smaller (Table 1). A significant influence of VPD on RRLP was detected in the stand at 1,050 m, but not at the other altitudes (Table 3). In the lowermost stand RRLP showed a strong positive correlation to mean VPD. In contrast, RRLL was not significantly related to the VPD level at any of the five sites (Table 3).
All investigated soil and climate parameters showed a significant influence on RRLP in the five stands (Table 4). A negative correlation existed with pH, air temperature and VPD, and a positive one to precipitation, soil moisture and C/N. When testing the influence of the parameters separately for the lower and upper transect sections (Table 5), it turned out that a given environmental factor typically influences fine root turnover at stands at lower and at higher elevations differently. In the stands between 1,050 and 1,890 m, turnover was positively affected by air temperature and VPD and negatively by soil moisture and soil C/N. At mid- and upper elevations (1,890–3,060 m) turnover was negatively related to air temperature, VPD and pH, while soil moisture and soil C/N showed positive effects.
Discussion
Elevational changes in fine root dynamics
Gill and Jackson (2000) examined the influence of climate on root turnover on a global scale and identified temperature as the most important variable for explaining patterns of root turnover. Despite high variability in data, a positive relationship between root turnover and mean annual temperature emerged. They suggest that an increase in temperature is associated with an increase of maintenance respiration, higher rates of nutrient mineralization and an increased pathogen and herbivore activity, which should decrease root lifespan. Empirical evidence in grassland and forest ecosystems supports the assumption that root turnover is higher under higher temperatures (e.g. Forbes et al. 1997; King et al. 1999). In the Ecuadorian transect, a temperature decrease by 3.7 K between 1,050 and 1,890 m a.s.l. in the lower transect resulted in a 40% decrease of annual root loss rate, which is best explained by a temperature effect on root turnover. However, other factors could be responsible as well. In fact, we found an opposite trend in root turnover rate towards higher altitudes. The absence of a linear altitudinal trend in fine root dynamics seems to indicate that temperature is only one of several factors influencing fine root turnover. Fitter et al. (1998) suggest that root growth is determined more by resource availability and source-sink relationship within the plant than temperature. In a study of an altitudinal gradient in temperate grasslands, these authors found root respiration to be correlated with radiation totals but not with temperature. This indicates that carbon supply is a major control for root activity in grasses and that high altitude species are well acclimated to low temperatures. In the upper part of the Ecuador transect (1,890–3,060 m) relative root loss increased by about 50%, suggesting that temperature effects on root turnover are overlaid by other influential factors despite a decrease in mean annual temperature of 6.3 K.
The growth of tropical montane forests has widely been found to be limited by the availability of nitrogen (Tanner et al. 1998; Benner et al. 2008). Several lines of evidence indicate that N availability decreases with elevation in South Ecuador, including topsoil C/N-ratios and foliar and root N concentrations (Röderstein 2006; Moser, unpublished). N deficiency is usually expected to increase fine root biomass and root longevity, and to decrease root turnover (Roy and Singh 1995; Eissenstat and Yanai 1997; Nadelhoffer 2000; Yavitt and Wright 2001; Powers et al. 2005) and the respiration costs of root maintenance (Eissenstat et al. 2000). However, opposite effects of nutrient availability on fine root longevity have been reported as well (Vogt et al. 1986; Pregitzer et al. 1993; Burton et al. 2000). Whether N deficiency decreases or increases root longevity may depend on the cost of maintaining a root as compared to the costs of allowing a root to die and to produce a new root (Van Noordwijk et al. 1998; Eissenstat et al. 2000). Roots may be maintained as long as the nutrients they provide outweigh the C cost of keeping the roots alive (Burton et al. 2000). It appears that in the high-elevation stands of Ecuador the costs for a tree in terms of C supply to the roots for extended periods are too high when related to the nutrients the roots provide. In those nutrient-poor soils it may be advantageous to produce new roots which are more efficient in nutrient uptake than older roots.
Tryon and Chapin (1983) found a reduced root elongation in boreal trees with decreasing temperature, and a large reduction in root growth below 5°C. Their findings also suggest a shift in carbon allocation from large exploratory roots to fine absorbing roots at low temperatures. In the uppermost stand (3,060 m) of the Ecuador transect, where night temperatures below 5°C may occur incidentally, the percentage of very fine roots with diameters <0.5 mm was relatively high. Thus, a high root turnover in this stand is mostly a consequence of a rapid shedding and regrowth of the very fine roots.
Another factor that may reduce root longevity at the highest altitude could be the very low pH value of the soil. Elevated concentrations of free aluminum associated with low pH were found to reduce root longevity in temperate forests (Raynal et al. 1990; Godbold et al. 2003). Godbold et al. (2003) reported increased fine root turnover in Norway spruce stands subjected to soil acidification. Soethe et al. (2007) found decreasing Mg concentrations in the fine root biomass with increasing altitude in the South Ecuadorian transect, which may be a consequence of Al/Mg antagonism in root uptake when Al concentrations are high.
Finally, oxygen deficiency may be an additional stressor reducing fine root longevity (Jackson and Ricard 2003) in the high elevation stands, where soil moisture is typically high and water logging may persist for extended periods.
In the absence of continuous high-resolution radiation and soil moisture measurements, we used VPD as a proxy of sunny-dry and clouded-wet periods. By correlation analysis we detected a positive influence of precipitation and soil moisture, and a negative one of VPD on root length production when the data of the entire transect are analyzed (Table 4). This indicates that, as a whole, root growth of the mountain forest trees is stimulated by a higher water availability which seems to act as a second controlling factor besides temperature. This is in accordance with results from tropical lowland forests in Malaysia and Panama, where root growth was higher in moist than in dry seasons (Green et al. 2005; Yavitt and Wright 2001). An exception was the lowermost stand at 1,050 m, which showed a positive effect of the VPD of the preceding month on fine root birth rate. We speculate that a higher VPD and thus a higher radiation input may have increased carbohydrate availability for root growth at this site, probably outweighing any drought-induced root losses.
Fine root turnover depending on root diameter
Studies on the morphological patterns of fine roots in tropical forests are very scarce, which is partly a consequence of the high species diversity of these forests which makes more general conclusions difficult. By analyzing fine root dynamics separately for the size classes <2.0 and <0.5 mm, a clear root size effect on root turnover and root longevity became evident, with thinner roots being more dynamic than thicker ones. Similarly, other authors also found this strong dependence of root survivorship on root diameter (Gill and Jackson 2000; Wells and Eissenstat 2001; King et al. 2002; Baddeley and Watson 2005). This may be explained by higher nutrient concentrations and respiration rates of small diameter roots, i.e. a generally higher metabolic activity (Pregitzer et al. 1997; Pregitzer et al. 1998; Withington et al. 2006). However, longevity also depends on branching order, as roots of high branching orders emerge later but die simultaneously with their carrier root (Majdi et al. 2001).
In our study it became obvious that at sites with highest fine root turnover (i.e. the sites at 1,050, 2,380, 3,060 m) the proportion of finest roots (diameter <0.5 mm) in overall fine root turnover was especially high. One may speculate that the fast replacement of finest roots is an adaptive mechanism under limiting environmental conditions, i.e. high temperatures at lower altitudes and nutrient limitation or adverse soil chemical conditions at higher altitudes. Manipulative studies have to reveal what type of exogenous or endogenous factors may be responsible for elevated root turnover rates at different sections of this elevation transect. In fact, root dynamics seem to be controlled by several factors along this 2,000-m elevation transect, which play different roles at different altitudes.
Conclusions
This transect study allows the conclusion that relatively high temperatures increase root turnover in the lower, rather hot and moist section of the transect. In contrast, at higher altitudes with a perhumid, cool climate and unfavorable soil fertility and acidity conditions, root longevity was probably restricted by the adverse soil physical and chemical environment. Thus, the U-shaped curve of fine root turnover rate along the slope is explained by different overlaying environmental gradients along the transect.
Abbreviations
- a.s.l.:
-
Above sea level
- d:
-
Diameter
- ECSF:
-
Estación Científica San Francisco
- RRLL:
-
Relative root length loss
- RRLP:
-
Relative root length production
- VPD:
-
Vapor pressure deficit
References
Aerts R, Berendse F, Bakker C (1989) Root production and root turnover in two dominant species of wet heathlands. Oecologia 81:374–378
Alexander IJ, Fairley RI (1983) Effects of N fertilization on populations of fine roots and mycorrhizas in spruce humus. Plant Soil 71:49–53
Baddeley JA, Watson CA (2005) Influences of root diameter, tree age, soil depth and season on fine root survivorship in Prunus avium. Plant Soil 276:15–22
Benner J, Vitousek P, Ostertag R (2008) Nutrient cycling and nutrient limitation in tropical montane cloud forest. In: Bruijnzeel S, Juvik J (eds) Mountains in the mist: science for conserving and managing tropical montane cloud forests. Hawaii Univ. Press, Honolulu (in press)
Buckland ST, Campbell CD, Mackie-Dawson LA, Horgan GW, Duff EI (1993) A method for counting roots observed in minirhizotrons and their theoretical conversion to root length density. Plant Soil 153:1–9
Burton AJ, Pregitzer KS, Hendrick RL (2000) Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 125:389–399
Cuevas E, Medina E (1988) Nutrient dynamics within Amazonian forests; II. Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76:222–235
Eissenstat DM, Yanai RD (1997) The ecology of root life span. Adv Ecol Res 27:1–62
Eissenstat DM, Wells CE, Yanai RD, Whitebeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Fitter AH, Graves JD, Self GK, Brown TK, Bogie DS, Taylor K (1998) Root production, turnover and respiration under two grassland types along an altitudinal gradient: influence of temperature and solar radiation. Oecologia 114:20–30
Forbes PJ, Black KE, Hooker JE (1997) Temperature-induced alteration to root longevity in Lolium perenne. Plant Soil 190:87–90
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Godbold DL, Fritz HW, Jentschke G, Meesenburg H, Rademacher P (2003) Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity. Tree Physiol 23:915–921
Green JJ, Dawson LA, Proctor J, Duff EI, Elston DA (2005) Fine root dynamics in a tropical rain forest is influenced by rainfall. Plant Soil 276:23–32
Hendrick RL, Pregitzer KS (1992) The demography of fine roots in a northern hardwood forest. Ecology 73:1094–1104
Hendrick RL, Pregitzer KS (1996) Applications of minirhizotrons to understand root function in forests and other natural ecosystems. Plant Soil 185:293–304
Hertel D, Leuschner C (2008) Fine root mass and fine root production in tropical moist forests as dependent on soil, climate and elevation. In: Bruijnzeel S, Juvik J (eds) Mountains in the mist: science for conserving and managing tropical montane cloud forests. Hawaii Univ. Press, Honolulu (in press)
Homeier J, Werner FA, Gradstein SR, Breckle S-W, Richter M (2008) Potential vegetation and floristic composition of Andean forests in South Ecuador, with a focus on the RBSF. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R (eds) Gradients in a tropical mountain ecosystem in Ecuador. Springer, Berlin, pp 87–100
Jackson MB, Ricard B (2003) Physiology, biochemistry and molecular biology of plant root systems subjected to flooding of the soil. In: de Kroon H, Visser EJW (eds) Root ecology. Springer, Berlin, pp 193–214
Jordan CF, Escalante G (1980) Root productivity in an Amazonian rain forest. Ecology 61:14–18
Joslin JD, Wolfe MH (1999) Disturbances during minirhizotron installation affect root observation data. Soil Sci Soc Am J 63:218–221
Joslin JD, Wolfe MH, Hanson PJ (2000) Effects of altered water regimes on forest root systems. New Phytol 147:117–129
King JS, Pregitzer KS, Zak DR (1999) Clonal variation in above- and below-ground growth responses of Populus tremuloides Michaux: influence of soil warming and nutrient availability. Plant Soil 217:119–130
King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty P (2002) Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol 154:389–398
Leuschner C, Moser G, Bertsch C, Röderstein M, Hertel D (2007) Large altitudinal increase in tree root/shoot ratio in tropical mountain forests of Ecuador. Basic Appl Ecol 8:219–230
Mainiero R, Kazda M (2006) Depth-related fine root dynamics of Fagus sylvatica during exceptional drought. For Ecol Manage 237:135–142
Majdi H (1996) Root sampling methods—applications and limitations of the minirhizotron technique. Plant Soil 185:255–258
Majdi H, Damm E, Nylund J-E (2001) Longevity of mycorrhizal roots depends on branching order and nutrient availability. New Phytol 150:195–202
Moser G, Röderstein M, Soethe N, Hertel D, Leuschner C (2008) Altitudinal changes in stand structure and biomass allocation of tropical mountain forests in relation to microclimate and soil chemistry. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R (eds) Gradients in a tropical mountain ecosystem in Ecuador. Springer, Berlin, pp 229–242
Nadelhoffer KJ (2000) The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol 147:131–139
Ostertag R (2001) Effects of nitrogen and phosphorus availability on fine-root dynamics in Hawaiian montane forests. Ecology 82:485–499
Powers JS, Treseder KK, Lerdau MT (2005) Fine roots, arbuscular mycorrhizal hyphae and soil nutrients in four neotropical rain forests: patterns across large geographic distances. New Phytol 165:913–921
Pregitzer KS, Hendrick RL, Fogel R (1993) The demography of fine roots in response to patches of water and nitrogen. New Phytol 125:575–580
Pregitzer KS, Kubiske ME, Yu CK, Hendrick RL (1997) Relationships among root branch order, carbon, and nitrogen in four temperate species. Oecologia 111:302–308
Pregitzer KS, Laskowski MJ, Burton AJ, Lessard VC, Zak DR (1998) Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol 18:665–670
Pregitzer KS, King JS, Burton AJ, Brown SE (2000) Responses of tree fine roots to temperature. New Phytol 147:105–115
Raynal DJ, Joslin JD, Thornton FC (1990) Sensitivity of tree seedlings to aluminum: III. Red spruce and loblolly pine. J Environ Qual 19:180–187
Richter M (2003) Using epiphytes and soil temperature for eco-climatic interpretations in south Ecuador. Erdkunde 57:161–181
Röderstein M (2006) Struktur und Dynamik des Feinwurzelsystems von tropischen Bergwäldern in Abhängigkeit von der Meereshöhe in Südecuador. Berichte des Forschungszentrums Waldökosysteme, Reihe A, Bd. 197. Ph.D. dissertation
Röderstein M, Hertel D, Leuschner C (2005) Above- and below-ground litter production in three tropical montane forests in southern Ecuador. J Trop Ecol 21:483–492
Roy S, Singh JS (1995) Seasonal and spatial dynamics of plant-available N and P pools and N-mineralization in relation to fine roots in a dry tropical forest. Soil Biol Biochem 27:33–40
Santantonio D, Grace JC (1987) Estimating fine-root production and turnover from biomass and decomposition data: a compartment-flow model. Can J For Res 17:900–908
Schrumpf M, Guggenberger G, Valarezo C, Zech W (2001) Tropical montane rain forest soils—development and nutrient status along an altitudinal gradient in the South Ecuadorian Andes. Erde 132:43–59
Silver WL, Thompson AW, McGroddy ME, Varner RK, Dias JD, Silva H et al (2005) Fine root dynamics and trace gas fluxes in two lowland tropical forest soils. Glob Change Biol 11:290–306
Soethe N, Lehmann J, Engels C (2007) Carbon and nutrient stocks in roots of forests at different altitudes in the Ecuadorian Andes. J Trop Ecol 23:319–328
Tanner EVJ (1981) The decomposition of leaf litter in Jamaican montane rain forests. J Ecol 69:263–273
Tanner EVJ, Vitousek PM, Cuevas E (1998) Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 79:10–22
Trumbore S, Salazar da Costa E, Nepstad DC, Barbosa de Camargo P, Martinelli LA, Ray D et al (2006) Dynamics of fine root carbon in Amazonian tropical ecosystems and the contribution of roots to soil respiration. Glob Change Biol 12:217–229
Tryon PR, Chapin FS (1983) Temperature control over root growth and root biomass in taiga forest trees. Can J For Res 13:827–833
Van Noordwijk M, Martikainen P, Bottner P, Cuevas E, Rouland C, Dhillion SS (1998) Global change and root function. Glob Change Biol 4:759–772
Vitousek PM, Sanford RL Jr (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Vogt KA, Grier CC, Vogt DJ (1986) Production, turnover, and nutrient dynamics of above and belowground detritus of world forests. Adv Ecol Res 15:303–377
Vogt KA, Publicover DA, Bloomfield J, Perez JM, Vogt DJ, Silver WL (1993) Belowground responses as indicators of environmental change. Environ Exp Bot 33:189–205
Wells CE, Eissenstat DM (2001) Marked differences in survivorship among apple roots of different diameters. Ecology 82:882–892
Withington J, Reich PB, Oleksyn J, Eissenstat DM (2006) Comparisons of structure and life span in roots and leaves among temperate trees. Ecol Monogr 76:381–397
Yavitt JB, Wright SJ (2001) Drought and irrigation effects on fine root dynamics in a tropical moist forest, Panama. Biotropica 33:421–434
Acknowledgements
The financial support granted by the German Science Foundation (DFG) through Research Group (Forschergruppe) 402 (subproject B6) is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Graefe, S., Hertel, D. & Leuschner, C. Fine root dynamics along a 2,000-m elevation transect in South Ecuadorian mountain rainforests. Plant Soil 313, 155–166 (2008). https://doi.org/10.1007/s11104-008-9688-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9688-z