Abstract
Key message
Hydrolysis of 1-octen-3-yl β-primeveroside implemented by a system with high structure-specificity is accountable for the rapid formation of 1-octen-3-ol from soybean leaves after mechanical wounding.
Abstract
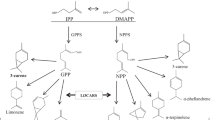
1-Octen-3-ol is a volatile compound ubiquitous in fungi; however, a subset of plant species also has the ability to form 1-octen-3-ol. Owing to its volatile nature, it has been anticipated that 1-octen-3-ol is associated with the effort of the emitter to control the behavior of the surrounding organisms; however, its ecological significance and the enzymes involved in its biosynthesis have not been fully elucidated, particularly in plants. We previously found that soybean (Glycine max) seeds contain 1-octen-3-yl β-primeveroside (pri). To elucidate the physiological significance and the biosynthesis of 1-octen-3-ol in plants, changes in the amount of 1-octen-3-yl pri during development of soybean plants was examined. A high 1-octen-3-yl pri level was found in young developing green organs, such as young leaves and sepals. Treatment of soybean leaves with methyl jasmonates resulted in a significant increase in the amount of 1-octen-3-yl pri; suggesting its involvement in defense responses. Although 1-octen-3-ol was below the detection limit in intact soybean leaves, mechanical damage to the leaves caused rapid hydrolysis of almost all 1-octen-3-yl pri to liberate volatile 1-octen-3-ol. Under the same conditions, the other glycosides, including isoflavone glycoside and linalool diglycoside, were hardly hydrolyzed. Therefore, the enzyme system to liberate aglycone from glycosides in soybean leaves should have strict substrate specificity. 1-Octen-3-yl pri might function as a storage form of volatile 1-octen-3-ol for immediate response against stresses accompanying tissue wounding.



Similar content being viewed by others
Data availability
Data available upon request.
Code availability
Not applicable.
References
Badenhop AF, Wilkens WF (1969) The formation of 1-octen-3-ol in soybeans during soaking. J Am Oil Chem Soc 46:179–182. https://doi.org/10.1007/BF02635729
Boggia L, Sgorbini B, Bertea CM, Cagliero C, Bicchi C, Maffei ME, Rubiolo P (2015) Direct contact-sorptive tape extraction coupled gas chromatography-mass spectrometry to reveal volatile topographical dynamics of lima bean (Phaseolus lunatus L.) upon herbivory by Spodoptera littoralis Boids. BMC Plant Biol 15:102. https://doi.org/10.1186/s12870-015-0487-4
Brash AR, Niraula NP, Boeglin WE, Mashhadi Z (2014) An ancient relative of cyclooxygenase in cyanobacteria is a linoleate 10S-dioxygenase that works in tandem with a catalase-related protein with specific 10S-hydroperoxide lyase activity. J Biol Chem 289:13101–21311. https://doi.org/10.1074/jbc.M114.555904
Clay DE, Carlson, CG, Clay SA, Wagner L, Deneke DL, Hay CH (2013) iGrow soybeans: best management practices for soybean production. Agronomy, horticulture, and plant science books. South Dakota State University, USA
Contreras-Cornejo HA, Marcías-Rodríguez L, del Val E, Larsen J (2019) The root endophytic fungus Trichoderma atroviride induces foliar herbivory resistance in maize plants. Appl Soil Ecol 124:45–53. https://doi.org/10.1016/j.apsoil.2017.10.004
Croisier E, Rempt M, Pohnert G (2010) Survey of volatile oxylipins and their biosynthetic precursors in bryophytes. Phytochemistry 71:574. https://doi.org/10.1016/j.phytochem.2009.12.004
Cui J, Katsuno T, Totsuka K et al (2016) Characteristic fluctuations in glycosidically bound volatiles during tea processing and identification of their unstable derivatives. J Agric Food Chem 64:1151–1157. https://doi.org/10.1021/acs.jafc.5b05072
Ferrari R, Lacaze I, Le Faouder P et al (2018) Cyclooxygenase and lipoxygenases are used by the fungus Podospora anserina to repel nematodes. Biochim Biophys Acta 1862:2174–2182. https://doi.org/10.1016/j.bbagen.2018.07.012
Fujita R, Yokono M, Ube N, Okuda Y, Ushijima S, Fukushima-Sakuno E, Ueno K, Osaki-Oka K, Ishihara A (2021) Suppression of Alternaria brassicicola infection by volatile compounds from spent mushroom substrates. J Biosci Bioeng 132:25–32. https://doi.org/10.1016/j.jbiosc.2021.03.003
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Ann Rev Plant Biol 57:303–333. https://doi.org/10.1146/annurev.arplant.57.032905.105228
Heiling S, Llorca LC, Li J, Gase K, Schmidt A, Schäfer M, Schneider B, Halitschke R, Gaquerel E, Baldwin IT (2021) Specific decorations of 17-hydroxygeranyllinalool diterpene glycosides solve the autotoxicity problem of chemical defense in Nicotiana attenuata. Plant Cell 33:1748–1770. https://doi.org/10.1093/plcell/koab048
Holighaus G, Rohlfs M (2019) Volatile and non-volatile fungal oxylipins in fungus-invertebrate interactions. Fungal Ecol 38:28–36. https://doi.org/10.1016/j.funeco.2018.09.005
Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW (2013) Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proc Natl Acad Sci USA 110:19561–19566. https://doi.org/10.1073/pnas.1318830110
Inamdar AA, Morath S, Bennett JW (2020) Fungal volatile organic compounds: more than just a funky smell? Ann Rev Microbiol 74:101–116. https://doi.org/10.1146/annurev-micro-012420-080428
Kataoka R, Watanabe T, Yano S, Mizutani O, Yamada O, Kasumi T, Ogihara J (2020) Aspergillus luchuensis fatty acid oxygenase ppoC is necessary for 1-octen-3-ol biosynthesis in rice koji. J Biosci Bioeng 129:192–198. https://doi.org/10.1016/j.jbiosc.2019.08.010
Khoja S, Eltayef KM, Baxter I, Myrta A, Bull JC, Butt T (2021) Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol Control 152:104472. https://doi.org/10.1016/j.biocontrol.2020.104472
Kigathi RN, Unsicker SB, Reichelt M, Kesselmeier J, Gershenzon J, Weisser WW (2009) Emission of volatile organic compounds after herbivory from Trifolium pratense (L.) under laboratory and field condition. J Chem Ecol 35:1335–1348. https://doi.org/10.1007/s10886-009-9716-3
Kihara H, Tanaka M, Yamato KT, Horibata A, Yamada A, Kita S, Ishizaki K, Kajikawa M, Fukuzawa H, Kohchi T, Akakabe Y, Matsui K (2014) Arachidonic acid-dependent carbon-eight volatile synthesis from wounded liverwort (Marchantia polymorpha). Phytochemistry 107:42–49. https://doi.org/10.1016/j.phytochem.2014.08.008
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2007) Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J General Plant Pathol 73:35–37. https://doi.org/10.1007/s10327-006-0314-8
Kobayashi A, Tsuda Y, Hirata N, Kubota K, Kitamura K (1995) Aroma constituents of soybean [Glycine max (L.) Merril] milk lacking lipoxygenase isozymes. J Agric Food Chem 43:2449–2452. https://doi.org/10.1021/jf00057a025
Leitner M, Kaiser R, Hause B, Boland W, Mithöfer A (2010) Does mycorrhization influence herbivore-induced volatile emission in Medicago trancatula? Mycorrhiza 20:89–101. https://doi.org/10.1007/s00572-009-0264-z
Ma SJ, Mizutani M, Hiratake J, Hayashi K, Yagi K, Watanabe N, Sakata K (2001) Substrate specificity of b-premeverosidase, a key enzyme in aroma formation during ooling tea and black tea manufacturing. Biosci Biotechnol Biochem 65:2719–2729. https://doi.org/10.1271/bbb.65.2719
Maeda R, Waki T, Kawai Y, Takahashi S, Nakayama T (2018) Involvement of chalcone reductase in the soybean isoflavone metabolon: identification of GmCHR5, which interacts with 2-hydroxyisoflavonone synthase. Plant J 96:56–74. https://doi.org/10.1111/tpj.14014
Matsui K, Takemoto H, Koeduka T, Ohnishi T (2018) 1-Octen-3-ol is formed from its glycoside during processing of soybean [Glycine max (L.) Merr.] seeds. J Agric Food Chem 66:7409–7416. https://doi.org/10.1021/acs.jafc.8b01950
Mochizuki S, Sugimoto K, Koeduka T, Matsui K (2016) Arabidopsis lipoxygenase 2 is essential for formation of green leaf volatiles and five-carbon volatiles. FEBS Lett 590:1017–1027. https://doi.org/10.1002/1873-3468.12133
Ohgami S, Ono E, Horikawa M et al (2015) Volatile glycosylation in tea plants: sequential glycosylations for the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol 168:464–477. https://doi.org/10.1104/pp.15.00403
Okull DO, Beelman RB, Gourama H (2003) Antifungal activity of 10-oxo-trans-8-decenoic acid and 1-octen-3-ol against Penicillium expansum in potato dextrose agar medium. J Food Prot 66:1503–1505. https://doi.org/10.4315/0362-028X-66.8.1503
Osei-Owusu J, Vuts J, Caulfield JC, Woodcock CM, Withall DM, Hooper AM, Osafo-Acquaah S, Birkett MA (2020) Identification of semiochemicals from cowpea, Vigna unguiculata, for low-input management of the legume pod borer, Maruca vitrata. J Chem Ecol 46:288–298. https://doi.org/10.1007/s10886-020-01149-7
Ozawa R, Shimoda T, Kawaguchi M, Arimura G, Horiuchi J, Nishioka Y, Takabayashi J (2000) Lotus japonicus infested with herbivorous mites emits volatile compounds that attract predatory mites. J Plant Res 113:427–433. https://doi.org/10.1007/PL00013951
Potter CJ (2014) Stop the biting: targeting a mosquito’s sense of smell. Cell 156:878–881. https://doi.org/10.1016/j.cell.2014.02.003
Quintana-Rodriguez E, Morales-Bargas AT, Molina-Torres J, Adame-Alvarez RM, Acosta-Gallegos JA, Heil M (2015) Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuithianum. J Ecol 103:250–260. https://doi.org/10.1111/1365-2745.12340
Quintana-Rodriguez E, Rivera-Macias LE, Adame-Alvarez RM, Torres JM, Heil M (2018) Shared weapons in fungus-fungus and fungus-plant interactions? Volatile organic compounds of plant or fungal origin exert direct antifungal activity in vitro. Fungal Ecol 33:115–121. https://doi.org/10.1016/j.funeco.2018.02.005
Senger T, Wichard T, Kunze S, Göbel C, Lerchl J, Pohnert G, Feussner I (2005) A multifunctional lipoxygenase with fatty acid hydroperoxide cleaving activity from the moss Physcomitrella patens. J Biol Chem 280:7588–7596. https://doi.org/10.1074/jbc.M411738200
Seo WH, Baek HH (2009) Characteristic aroma-active compounds of Korean perilla (Perilla f rutescens Britton) leaf. J Agric Food Chem 57:11537–11542. https://doi.org/10.1021/jf902669d
Sugimoto K, Matsui K, Iijima Y et al (2014) Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc Natl Acad Sci USA 111:7144–7149. https://doi.org/10.1073/pnas.1320660111
Wang T, Wang S, Wang Y, Li J, Yan F, Liu Y, Zhao L, Wang Q (2020) Jasmonic acid-induced inhibition of root growth and leaf senescence is reduced by GmbHLH3, a soybean bHLH transcription factor. Can J Plant Sci. https://doi.org/10.1139/cjps-2019-0250
Wang R, Ren C, Dong S, Chen C, Xian B, Wu Q, Wang J, Pei J, Chen J (2021) Integrated metabolomics and transcriptome analysis of flavonoid biosynthesis in safflower (Carthamus tinctorius L.) with different colors. Front Plant Sci. https://doi.org/10.3389/fpls.2021.712038
Wasternack C, Strnad M (2019) Jasmonates are signals in the biosynthesis of secondary metabolites—pathways, transcription factors and applied aspects—a brief review. New Biotechnol 48:1–11. https://doi.org/10.1016/j.nbt.2017.09.007
Wu SY, Duncan LW (2020) Recruitment of an insect and its nematode natural enemy by olfactory cues from a saprophytic fungus. Soil Biol Biochem 144:107781. https://doi.org/10.1016/j.soilbio.2020.1007781
Wurzenberger M, Grosch W (1982) The enzymic oxidative breakdown of linoleic acid in mushrooms (Psalliota bispora). Z Lebensm Unters Forsch 175:186–190. https://doi.org/10.1007/BF01139769
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was partly supported by the financial support from Japan Society for the Promotion of science (JSPS) KAKENHI [16H02887 and 19H02887] to K.M.
Author information
Authors and Affiliations
Contributions
Experiment were designed by JMN and KM, and mostly conducted by JMN. LC-MS/MS analysis of glycosides of monoterpene alcohols was performed by FK and TO. The manuscript was written by JMN, TK, and KM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Consent to participate
All authors agree.
Consent for publication
All authors agree.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11103_2021_1226_MOESM2_ESM.pptx
Supplementary file2 Supplemental Fig. S1. Effect of exposure of intact soybean leaves with linalool vapor on the chromatograms of linalyl diglycoside. A. Chromatogram of unexposed leaves. B. Chromatogram of linalool-exposed leaves. Only the peak at a retention time of 16.0 (corresponding to peak a in Fig. 3) increased after exposure (PPTX 112 kb)
Rights and permissions
About this article
Cite this article
Ntoruru, J.M., Ohnishi, T., Katsumata, F. et al. 1-Octen-3-ol is formed from its primeveroside after mechanical wounding of soybean leaves. Plant Mol Biol 109, 551–561 (2022). https://doi.org/10.1007/s11103-021-01226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01226-9