Abstract
Symbiosis with mycorrhizal fungi substantially impacts secondary metabolism and defensive traits of colonised plants. In the present study, we investigated the influence of mycorrhization (Glomus intraradices) on inducible indirect defences against herbivores using the model legume Medicago truncatula. Volatile emission by mycorrhizal and non-mycorrhizal plants was measured in reaction to damage inflicted by Spodoptera spp. and compared to the basal levels of volatile emission by plants of two different cultivars. Emitted volatiles were recorded using closed-loop stripping and gas chromatography/mass spectrometry. The documented volatile patterns were evaluated using multidimensional scaling to visualise patterns and stepwise linear discriminant analysis to distinguish volatile blends of plants with distinct physiological status and genetic background. Volatile blends emitted by different cultivars of M. truncatula prove to be clearly distinct, whereas mycorrhization only slightly influenced herbivore-induced volatile emissions. Still, the observed differences were sufficient to create classification rules to distinguish mycorrhizal and non-mycorrhizal plants by the volatiles emitted. Moreover, the effect of mycorrhization turned out to be opposed in the two cultivars examined. Root symbionts thus seem to alter indirect inducible defences of M. truncatula against insect herbivores. The impact of this effect strongly depends on the genetic background of the plant and, hence, in part explains the highly contradictory results on tripartite interactions gathered to date.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonisation of a plant with arbuscular mycorrhizal (AM) fungi is associated with drastic changes of the physiology and ecology of the plant. Out of the multitude of effects produced by AM fungi, two constituted the background of this study: Plants associated with AM fungi undergo radical changes in secondary metabolism and become more resistant to all kinds of pests, pathogens as well as phytophages. A connection between these phenomena is easily conceivable, i.e. altered production of defensive compounds may lead to changes in plant resistance.
Numerous effects on secondary metabolism have been documented so far. For example, in Medicago truncatula Gaertn. and Medicago sativa L., patterns of flavonoid accumulation and correlating biosynthetic enzymes change during the establishment of mycorrhizal symbiosis (Harrison and Dixon 1993). Extensive metabolite profiling of soluble secondary metabolites from mycorrhizal roots of M. truncatula revealed a number of metabolic characteristics, among them the stimulation of the biosynthesis of various isoflavonoids (Schliemann et al. 2008). In M. sativa, those changes have been shown to depend not only on the temporal progression of the interaction but also on the fungal species involved (Larose et al. 2002). Accumulation of cell-wall-bound phenolics (Grandmaison et al. 1993), triterpenoids (Akiyama and Hayashi 2002) and hydroxycinnamic acid amides (Peipp et al. 1997) has been found to be induced by AM fungi in different plant species. Moreover, the continuous accumulation of apocarotenoids in AM roots seems to be a widespread phenomenon (Fester et al. 2002). Finally, jasmonates, which are often associated with changes in secondary metabolism, and related biosynthetic enzymes occur at elevated levels in arbuscule-harbouring cells (Hause et al. 2007).
Though most of these reports concentrated on changes that occur in the roots, changes in secondary metabolites in aboveground plant parts have also been described. For example, changes in the content of essential oils have been reported in three genotypes of oregano (Origanum vulgare L.) and in Ocimum basilicum L. var. Genovese (Copetta et al. 2006; Khaosaad et al. 2006). Furthermore, mycorrhization of Citrus jambhiri Lush leads to an induced accumulation of leaf sesquiterpenoid volatiles (cited by Strack et al. 2003).
Mycorrhization also seems to entail a certain degree of bioprotection against different biotic and abiotic stresses. The interaction of mycorrhization with herbivores has been studied in depth, but the results so far are still quite inconsistent. As summarised by Gehring and Whitham (2002), some chief motifs can be stated: In the majority of cases studied, aboveground herbivores reduce mycorrhizal colonisation and alter the mycorrhizal fungi community composition, which could be due to the reduced ability of the attacked plant to supply the fungus with carbohydrates. However, belowground herbivores have been reported to facilitate fungal colonisation of Agrostis capillaris (Currie et al. 2006). Conversely, influences of mycorrhizal fungi on the performance of aboveground herbivores have been observed. The quality of this impact ranges from positive over neutral to negative. This substantial variation can to some extent be attributed to the species involved in the interaction, including fungal and herbivore species. For example, the performance of chewing and leaf-mining insects is predominantly negatively affected by AM symbiosis of the host plant, whereas sucking insects seem to profit from this interaction (Gange and West 1994; Hoffmann et al. 2009); counter-examples, however, also exist (Goverde et al. 2000). Another tendency indicates that AM plants are favourable for specialist herbivores and detrimental for generalists. Although this effect was observed for chewing herbivores, it was not detected for sucking herbivores (Gange et al. 2002). The complexity and obvious species specificity of these interactions have concisely been summarised by Gehring and Bennett (2009) and led to a call for stronger consideration of community approaches. Numerous studies investigated the impact of variations in fungal or herbivore species composition, but only few inquiries considered the influence of the host plant in these interactions. Moreover, the ecological net of interactions is not limited to fungus, plant and herbivore, but extends, amongst others, to natural enemies of the herbivore. These predators can be attracted to infested plants by the induced emission of volatile organic compounds (VOCs); this reaction thus represents a mechanism of indirect defence that functions via the recruitment of a higher trophic level to support plant defence (Mithöfer et al. 2009). Regarding the abovementioned complexity of tripartite interactions, it seems obvious to suggest that root symbionts could also affect predators and parasitoids of herbivores. This hypothesis has been tested by assessing effects of mycorrhization on the attractiveness of tomato plants to the parasitic wasp Aphidius ervi (Guerrieri et al. 2004). Mycorrhizal plants prove to attract significantly more wasps than control plants, whereas parasitoids could not discriminate between infested non-mycorrhizal and uninfested mycorrhizal plants. However, neither have infested non-mycorrhizal plants been compared to infested mycorrhizal plants, nor have the volatiles released been analysed (Guerrieri et al. 2004). Another study revealed that in field experiments parasitism of herbivores was reduced on mycorrhizal Leucanthemum vulgare Lam. plants (Gange et al. 2003). In the lab, this effect turned out to be strongly dependent on the fungal species associated with the plant. Both herbivore damage to the plant and parasitism on the herbivore were either reduced or remained unchanged by AM fungi, depending on the fungal species involved in the interaction (Gange et al. 2003). But consistently, AM symbiosis did not improve the searching efficiency of the parasitoid, which contrasts the data reported by Guerrieri et al. (2004). It has been reasoned that the observed effect could be due to increased plant size, which may impede the search of the parasitoid for its host (Gange et al. 2003). In this case, however, changes in induced volatile patterns as an effect of mycorrhization have not been considered as explanation.
Other mutualists of plants have also been shown to be affected by the symbiotic state of the plant, as pollinators were more strongly attracted to flowers of mycorrhizal fireweed (Chamerion angustifolium L.) than to those of non-mycorrhizal fireweed (Wolfe et al. 2005). In this study, it has been argued that the observed effect could correlate with the development of larger inflorescences by mycorrhizal plants, because other floral traits, such as nectar production or composition, were not influenced in this species. Again, the putative impact of changes in volatile emission has not been examined. So far, only one study assessed the impact of mycorrhization on VOC accumulation and emission (Rapparini et al. 2008). Some subtle effects were detectable, but the impact of herbivory has not been determined. In regard to these heterogeneous reports on the impact of AM symbiosis on plant–herbivore and multitrophic interactions, this study aimed to further investigate what components could contribute to the effects observed. Particularly, the influence of the host plant has so far largely been disregarded. In order fill this gap, the release of VOCs was assessed in non-mycorrhizal and mycorrhizal M. truncatula Gaertn. plants. This species provides a feasible model, as diverse cultivars with differing resistance traits are available, and a variety of VOCs has been found to be emitted in response to diverse stimuli. Both the emission by intact plants and the emission by plants damaged by generalist lepidopteran larvae (Spodoptera spp.) were monitored. Whether mycorrhization alone could change volatile profiles of undamaged plants or whether the VOC pattern was altered when induction by herbivore attack occurred was the focus of the investigation. Furthermore, two different cultivars of M. truncatula were used in this study to evaluate the influence of subtle changes in the host, yet another possible reason for the inconsistencies of previous reports. Finally, measured VOC blends were used to assess whether classification and prediction of volatile patterns are possible, and thus may serve as indicators of the physiological state of the plant.
Materials and methods
Plant material and mycorrhization
Two different batches of M. truncatula Gaertn. were used in the experiments. Seeds of M. truncatula cv. Jemalong A17 were kindly provided by J.M. Prosperi (INRA-SGAP, Montpellier, France). The second batch of seeds was obtained from AustraHort Pty Ltd (Cleveland, Australia) with a mixture of cultivars, including M. truncatula cv. Paraggio and cv. Jemalong (without further specification of the line). Given the clear phenotypic differences between these cultivars, we deem justifiable to assume that only plants of cv. Paraggio were used in the experiments presented. The clear differences between induced volatile emissions of the two batches of plants used support this view (cf. “Results”—“Visualisation of cultivar-specific differences”).
Seeds were sterilised in H2SO4, rinsed several times in water, transferred to moist filter paper, and kept at 4°C for 3 days. Afterwards, they were allowed to germinate in the dark for 1 day before being transferred to the greenhouse at 22°C. After 5 days, seedlings (five plants per 500 ml pot) were inoculated with the AM fungus G. intraradices Schenk & Smith isolate 49 (Maier et al. 1995) by transferring into expanded clay (Lecaton, 2–5 mm particle size; Fibo Exclay, Pinneberg, Germany) containing 15% (v/v) inoculum. The inoculum was enriched in propagules by cultivation with mycorrhizal leek (Allium porrum cv. Elefant). As non-mycorrhizal controls, plants were mock-inoculated with the same amount of inoculum, which was sterilised by autoclaving. Plants were grown at 23–25°C with a light–dark rhythm of 16/8 h and supplied once per week with 10 ml per pot of tenfold concentrated Long Ashton nutrient solution (Hewitt 1966), but containing only 20% phosphate. Four weeks after inoculation, the degree of mycorrhization was determined microscopically with the gridline intersection method after staining with trypan blue (Phillips and Hayman 1970) and revealed approximately 36 ± 21.5% and 59 ± 13.4% of total root length colonisation for M. truncatula cv. Paraggio and cv. Jemalong A17, respectively. At time of feeding experiments, both cultivars exhibited a mycorrhization rate of 66 ± 19.8% and 90 ± 4.2%, respectively. Controls (mock-inoculated) plants did not exhibit any AM structures in their roots.
Herbivores and infestation of plants
All plants used in the feeding experiments were between eight and 11 (cv. Paraggio) and eight and nine (cv. Jemalong A17) weeks old. In all experiments, either larvae of Spodoptera littoralis (Boisduval, 1833) or Spodoptera exigua (Hübner, 1808) were used. No differences between the volatile profiles induced by these species were found in M. truncatula. The larvae were kept on an artificial diet (500 g chopped beans, 9 g ascorbic acid, 9 g 4-ethylbenzoic acid, 0.7 g vitamin E and 4 ml formaldehyde per litre water are mixed with approximately 650 ml of a 7.5% agar solution) at 23°C with a light period from 7 a.m. to 9 p.m. For induction of volatile emission, ten to 15 larvae were allowed to feed on five M. truncatula plants in one pot for 48 h, i.e. during the entire time of volatile collection.
Analysis of VOC emission
Volatiles were collected over a period of 48 h using the closed-loop stripping method as described by Donath and Boland (1995). Volatiles were collected separately from nine to ten pots containing five plants each for each treatment group. These groups were: (a) non-mycorrhizal control plants (NM), (b) non-mycorrhizal plants damaged by Spodoptera spp. larvae (NM-W), (c) undamaged mycorrhizal plants (M), and (d) mycorrhizal, herbivore-infested plants (M-W). Pots were transferred to desiccators and connected to a circulation pump containing a charcoal trap (1.5 mg of charcoal, CLSA-Filter, Le Ruisseau de Montbrun, Daumazan sur Arize, France). Desorption was done using methylene chloride (2 × 20 µl) containing 100 µg ml−1 n-bromodecane as internal standard; the volatiles were analysed using gas chromatography/mass spectrometry (GC–MS; TRACE 2000 series, Finnigan, UK) equipped with an EC-5 capillary (Alltech, Unterhaching, Germany).
Compounds were identified according to their fragmentation pattern (MS) and by comparison of linear retention indices (RI). To compare mass spectra, the NIST/EPA/NIH Mass Spectral Library (Version 1998) and MassFinder (V 3.5; Dr. D. Hochmuth, Hamburg, Germany) were used. The measured retention indices were compared either with those calculated from pure reference compounds or with literature data (Adams 2001; Linstrom and Mallard 2005, and references therein; retention indices of the compound in question measured under comparable conditions, i.e. temperature ramp, equivalent GC column etc.). Deviations of ±2 for reference compounds and ±5 for literature data were accepted for identical compounds in accordance with Hochmuth (2004). Unidentified compounds were classified by their linear retention index on an EC-5 column under temperature-programmed conditions.
Relative quantification of the compounds emitted was done by relating the respective peak areas to that of the internal standard (100 µg ml−1 n-bromodecane). As only undamaged plants remaining in their substrate were used for analysis, the fresh weight could not be determined. However, the variation of volatile emission caused by differing biomasses can be assumed to be comparatively low, as all plants used were of approximately the same age and did not exhibit marked differences in development.
Statistical analyses
For all statistics on VOC emission, square root-transformed data were used. The first line of statistical analysis was done using one-way ANOVA combined with the Newman–Keuls post hoc test to compare the levels of single compounds emitted after the different treatments.
In order to achieve exploratory mappings of different treatment effects in a mathematical space as defined by the volatile blends, a non-metric multidimensional scaling (NMDS) ordination was carried out (Kruskal 1964) as detailed by Leitner et al. (2008). The analysis was performed in the way recommended by Minchin (1987), using the R package VEGAN (Oksanen et al. 2006). Prior to analysis, the data were standardised using square root transformation and tested for proper standardisation using the Shapiro–Wilk test of normality (Royston 1982). For analysis by NMDS, Euclidean distance was selected as dissimilarity measure. The overall goodness of fit of the models was measured by the stress statistic, the correlation between fitted values and ordination distances (Venables and Ripley 2002).This statistic gives the proportion of data not ideally depicted in the ordination.
As volatile blends proved to exhibit rather distinct patterns in NMDS, further approaches aimed to test whether certain treatments can be diagnosed by their respective volatile profiles.
Linear discriminant analysis (LDA) aims to find linear transformations of the variables that yield maximal separation of the given groups by maximising between-class variance and minimising within-class variance (McLachlan 1992). In doing so, it helps to describe differential features of observations, to sort objects into labelled classes and to assign new observations to previously defined classes. For simple LDA, multicollinearity poses a major problem. This term refers to multiple variables, which carry basically the same information—i.e. variables that are linearly correlated. In such instances, it cannot be determined which of the variables accounts for the variance of the dependent variable (group). This obstacle can be overcome by using stepwise selection of variables for classification. Here, forward variable selection was performed using the Wilk’s lambda criterion, as executed by the R package klaR (Mardia et al. 1979; Weihs et al. 2005). The value of Wilk’s lambda, which can range from 0 to 1, indicates whether the means between combinations of variables are different between groups. The smaller this value is, the more likely it is that the group means differ. The method used selects variables that minimise the Wilk’s lambda as long as the p value still indicates statistical significance at the 0.9 level.
The quality of the resulting models is usually given by the estimated error of misclassification. This parameter was tested by tenfold cross-validation. For that purpose, the actual measurements were split into a training set and a test set. The first is used to build up a classification rule, which in turn is used to predict the items in the test set, i.e. to allocate them to the appropriate group. This procedure is performed on ten different subsets (Venables and Ripley 2002); in order to achieve numerical stability, this test was repeated 99 times. The overall results, which give the proportions of classification and the error rates of the model in question, were summarised in a confusion matrix. Visual inspection of the model can be done by plotting the first few linear disciminants, i.e. the transformed variables.
Results
As previously demonstrated, M. truncatula reacts to herbivory by emitting a great variety of volatile compounds (Leitner et al. 2005). The aim of this study was to determine whether mycorrhization (with G. intraradices Schenck & Smith) influences this component of indirect defence. The impact of slight variations in the plant’s genetic background was considered as well, by including two different cultivars in the analysis.
VOC emission by M. truncatula cv. Jemalong A17
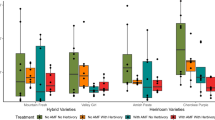
An overview on induced volatile emission of M. truncatula cv. Jemalong A17 (Fig. 1) demonstrates that caterpillar feeding induced the emission of a variety of compounds compared to undamaged plants, whereas differences between mycorrhizal and non-mycorrhizal plants are comparatively inconspicuous. Herbivore damage induced the emission of several VOCs, in which sesquiterpenoids represented the group of compounds most abundantly present. The overall pattern of VOC emission was quite similar in mycorrhizal and non-mycorrhizal plants for both damaged and undamaged plants. Most changes in the volatile pattern could be attributed to herbivore attack. However, some differences between mycorrhizal and non-mycorrhizal plants could be observed. These variations mainly concerned substances found in rather low abundance or even only trace amounts, with exception of two compounds that have not yet been identified. Though we were able to resolve the empirical formulae by high resolution mass spectrometry, the amounts collected were too low for full structural elucidation. These substances exhibited an emission pattern that specifically changed with mycorrhization. One of them, RI 1038 (C9H14O3), was emitted at significantly higher levels in mycorrhizal plants independent of wounding, whereas the emission of RI 1112 (C11H18O3) was clearly reduced in mycorrhizal plants (Fig. 2). For all other compounds, whose release seemed to be somehow influenced by mycorrhization, the overall tendency was a reduced emission of the respective compounds by mycorrhizal plants in reaction to herbivory. The compounds affected were α-gurjunene, α-himachalene, E-nerolidol, dihydroactinidiolide and 3,5-dimethoxytoluene (representing this pattern, the relative abundance of α-gurjunene emitted is shown in Fig. 2). Only n-tridecane showed a slight trend to elevated emission after herbivore attack in mycorrhizal plants.
Box plots representing relative quantification of volatiles emitted by non-mycorrhizal and mycorrhizal Medicago truncatula cv. Jemalong A17 plants in reaction to herbivory. NM control (non-mycorrhizal), unwounded; NM-W control, damaged by Spodoptera sp; M mycorrhizal, unwounded; M-W mycorrhizal, wounded by Spodoptera sp. n = 9–10. Small letters indicate significant differences (p < 0.05) between the different treatments as determined by ANOVA and Newman–Keuls post hoc test. Abbreviations: unidentified compounds are given by their respective retention index (RI); DMNT, 4,8-dimethylnona-1,3,7-triene; TMTT, (3E,7E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene; 2-s-But-3-methoxypyr, 2-sec-butyl-3-methoxypyrazine
Examples of volatile compounds emitted by Medicago truncatula cv. Jemalong A17, whose release changed upon mycorrhization. NM control (non-mycorrhizal), unwounded; NM-W control, wounded by Spodoptera sp.; M mycorrhizal, unwounded; M-W mycorrhizal, wounded by Spodoptera sp. n = 9–10. Small letters indicate significant differences (p < 0.05) as determined by ANOVA and Newman–Keuls post hoc test
VOC emission by M. truncatula cv. Paraggio
Besides M. truncatula cv. Jemalong A17, cv. Paraggio was used for experiments paralleling those described above (Fig. 3). First of all, it was striking that some clear discrepancies in the induced VOC patterns of the two batches of plants could be observed regardless of the symbiotic state of the plants (Figs. 1 and 3, cf. section “Visualisation of cultivar-specific differences”). These variations, however, did not affect the main components that were found to be induced by herbivory. But while no monoterpenoids were found to be induced in the cultivar Jemalong A17 at all, a considerable amount of linalool and low levels of limonene were emitted by cv. Paraggio. Also, the blend of sesquiterpenoids detected differed. Moreover, in Jemalong A17, all compounds that seemed to be affected by mycorrhization were released to a lower extent after caterpillar feeding, whereas it was the opposite in the case of cv. Paraggio. The emission of several sesquiterpenoids, such as β-copaene, α-himachalene, RI 1457, germacrene D and γ-muurolene (not thoroughly separable under the GC conditions used, thus quantified together as RI 1479), and 2-sec-butyl-3-methoxypyrazine (Fig. 4) turned out to be induced more strongly in mycorrhizal caterpillar-damaged plants than in non-mycorrhizal ones. Still, one clear parallel regarding the effect of mycorrhization was found in the emission patterns of RI 1038 and RI 1112 (Fig. 4). These substances showed the same increase or reduction, respectively, in reaction to mycorrhization as observed with the cultivar Jemalong A17. The characteristics were, however, not very pronounced, as drastic deviations occurred in some samples (excluded from statistical analysis).
Box plots representing relative quantification of volatiles emitted by non-mycorrhizal and mycorrhizal Medicago truncatula cv. Paraggio plants in reaction to herbivory. NM control (non-mycorrhizal), unwounded; NM-W control, damaged by Spodoptera sp; M mycorrhizal, unwounded; M-W mycorrhizal, wounded by Spodoptera sp. n = 7–10. Small letters indicate significant differences (p < 0.05) between the different treatments as determined by ANOVA and Newman–Keuls post hoc test. Abbreviations: unidentified compounds are given by their respective retention index (RI); RI 1479 combined, combined quantification of germacrene D and γ-muurolene; DMNT, 4,8-dimethylnona-1,3,7-triene; TMTT, (3E,7E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene; 2-s-But-3-methoxypyr, 2-sec-butyl-3-methoxy-pyrazine; MeJA, methyl jasmonate; MeSA, methyl salicylate
Examples of volatile compounds emitted by Medicago truncatula cv. Paraggio, whose release changed upon mycorrhization. NM control (non-mycorrhizal), unwounded; NM-W control, wounded by Spodoptera sp.; M, mycorrhizal, unwounded; M-W, mycorrhizal, wounded by Spodoptera sp. n = 7–10. Small letters indicate significant differences (p < 0.05) as determined by ANOVA and Newman–Keuls post hoc test
Visualisation of cultivar-specific differences
In view of the obvious differences between the VOC blends emitted by the different cultivars as outlined above, it was interesting to analyse the influence of the plants’ genetic background (Fig. 5a, b). In order to create a concise representation of these differences, VOC patterns were visualised by means of NMDS. This exploratory method of multivariate statistics aims to map objects (samples) in a low-dimensional space so that the resulting representation reproduces the original distances or similarities as closely as possible. The distances between any data point in the ordination thus indicate the respective similarities or correlations. In contrast to multidimensional scaling, NMDS uses rank orders instead of original similarities for scaling.
Non-metric multidimensional (NMDS) plots of VOC blends emitted by different Medicago truncatula cultivars in response to mycorrhization and feeding by Spodoptera sp. using square root-transformed data and Euclidean distance as a dissimilarity measure. a Volatiles emitted by unwounded plants; stress, 15.54. b Volatiles emitted in response to feeding by Spodoptera sp.; stress, 16.20. Open circles indicate the relative location of single volatile samples in ordination space. The size of the circles represents the goodness of the fit into the model for each particular sample. The centroids are given by the intersection of the spiderweb-like lines within each treatment group; the groups’ standard errors are given by filled ellipses. The size of the ellipse can be interpreted as a measure of consistency for the respective group. The relative distances between any sample or substance shown represent similarities and positive (low distances) or negative (high distances) correlations, respectively. Variables are plotted according to their weighted averages of ordination scores (samples) in which they occur
Regarding undamaged plants of both cultivars, including mycorrhizal and non-mycorrhizal plants, no compelling differences in the basal levels of VOC emission could be observed (Fig. 5a). However, when plants were damaged by lepidopteran larvae, the induced VOC blends of the two cultivars tested differed drastically (Fig. 5b). The differences between mycorrhizal and non-mycorrhizal plants proved to be minor, with some slight changes in cv. Jemalong A17; the different cultivars, however, could be easily separated by means of the induced VOC blends.
Still, no distinction between mycorrhizal and non-mycorrhizal plants could be depicted. In the results presented above, NMDS was calculated using Euclidean distance as a dissimilarity measure. Binomial NMDS led to the same results, as there were no qualitative differences between the VOC blends of mycorrhizal and non-mycorrhizal plants (data not shown). Nevertheless, from the basic data, it was obvious that at least slight influences are perceivable in the quantitative aspect. That these discrepancies could not be depicted using NMDS is unsurprising. This exploratory method aims only to depict complex patterns in a low-dimensional space, so that the interitem distances between any points represent the original similarities (or dissimilarities) observed as well as possible. Thus, NMDS is a way to visualise multivariate patterns and does not search for any disparities. Its strengths are the concise summaries produced and the plain algorithm, but this method does not place emphasis on minor differences. For this purpose, classification methods need to be applied.
Classification of VOC blends
In order to pinpoint the dissimilarities between VOC blends emitted by mycorrhizal and non-mycorrhizal plants, LDA was tested for its applicability to this problem. In contrast to NMDS, the underlying algorithm of LDA tries to produce a low-dimensional representation of the data that yields maximal separation of the given groups; thus, it serves to describe differences between certain groups (McLachlan 1992; Venables and Ripley 2002). However, LDA does not perform very well on multicollinear data. Multicollinearity refers to the fact that a range of variables, i.e. compounds detected, carry the same information, as for example several sesquiterpenes show highly similar emission patterns in response to herbivory. This problem can be overcome by eliminating collinear variables, for instance using stepwise classification. It is important to note that with this approach, the biological relevance of complete VOC blends remains in the background. Rather, this method emphasises diagnostic criteria for the distinct groups. Hence, the resulting rules of classification are to some extent replaceable, as only parts of the data are considered. In fact, several models with approximately the same level of significance have been calculated; in the following, only two examples will be presented, one for each batch of plants; in both cases, selection of variables was performed using the Wilk’s lambda as criterion.
For the cultivar Jemalong A17, ten out of 32 compounds were selected; namely, α-copaene, RI 1112, α-gurjunene, RI 1457, 7βH-silphiperfol-5-ene, geranylacetone, 7αH-silphiperfol-5-ene, RI 1038, n-tridecane and n-pentadecane. Using those variables as predictors, distinct grouping of all treatment classes can be achieved (Fig. 6). The resulting model was statistically significant with a Wilk’s lambda of 0.011 and an overall p value <0.0001 (8.42−14). Evaluation of the model using cross-validation revealed an error rate of 11.41% (Fig. 6). The proportion of classification, i.e. which samples were allocated to which group during cross-validation, can be read from a confusion matrix (Fig. 6). All groups could be predicted with reasonable success; errors occurred predominantly in the classification of undamaged, non-mycorrhizal plants.
Results of linear discriminant analysis for volatile blends emitted by Medicago truncatula cv. Jemalong A17 in response to mycorrhization and feeding by Spodoptera sp. The table represents the proportions of classification as determined by cross-validation (left). For visual inspection of the grouping, the first two linear discriminants were plotted (right)
In the case of cv. Paraggio, classification could be achieved with only six out of 31 compounds (Fig. 7). The VOCs selected were 2-sec-butyl-3-methoxypyrazine, β-caryophyllene, RI 1112, RI 1038, MeSA and 1-tetradecene. To test the level of significance of the model, the same analyses as those used for cv. Jemalong A17 were performed. With a value for Wilk’s lambda of 0.023 and an overall p value <0.0001 (2.19−13), this model also proves to be significant. The error rate, as determined by cross-validation, was 11.36% (Fig. 7). Misclassification occurred mainly between non-mycorrhizal unwounded and wounded plants and between unwounded mycorrhizal and non-mycorrhizal plants (Fig. 7), whereas mycorrhizal wounded plants could clearly be discriminated.
Results of linear discriminant analysis for volatile blends emitted by Medicago truncatula cv. Paraggio in response to mycorrhization and feeding by Spodoptera sp. The table represents the proportions of classification as determined by cross-validation (left). For visual inspection of the grouping, the first two linear discriminants were plotted (right)
Taken together, these results indicate that AM fungi do influence herbivore-induced VOC emission, though not very conspicuously. Still, these slight differences are sufficient to create classification rules and to distinguish plants with distinct physiological status. Thus, in this instance, VOC blends can indeed be used as diagnostic criteria. Strikingly, only a small proportion of the compounds detected was sufficient to build up rules of classification.
Discussion
In this study, we were able to demonstrate that herbivore-induced volatile emission differs strongly between different cultivars of M. truncatula, and that this component of indirect defence is influenced by symbiosis with AM fungi.
The former finding is not surprising, as cultivar-dependent variation in induced volatile emission has been described in other species as well (Takabayashi et al. 1991; Loughrin et al. 1995; Gouinguené et al. 2001; Degen et al. 2004). However, some striking differences between the two M. truncatula cultivars analysed were detected. The VOCs released constitutively by healthy plants did not differ conspicuously, but the blends emitted in response to herbivory were clearly distinct in the different cultivars. Although the main herbivore-inducible sesquiterpenoids were emitted in a similar manner, several compounds could be detected that were present only in one of the cultivars. It was particularly striking that no compounds derived from the methyl–erythritol phosphate pathway could be found in cv. Jemalong A17, whereas both monoterpenoids and diterpenoids were detected in VOC blends emitted by cv. Paraggio.
Overall, the effects of mycorrhization on VOC emission were not very prominent. Although no qualitative changes could be detected, a certain divergence in the quantitative aspect was recorded. Whereas in cv. Jemalong A17 some of the herbivore-induced VOCs were reduced in mycorrhizal plants, plants of cv. Paraggio tended to emit higher amounts of certain VOCs when mycorrhizal. In this context, it is remarkable to find in cultivar descriptions that cv. Paraggio is generally more resistant to all kinds of stresses than cv. Jemalong (Nair et al. 2006). For example, cv. Jemalong is susceptible to different species of aphids and abiotic stressors like boron, whereas cv. Paraggio at least moderately resists all those stresses. Since plants in their natural environment are usually mycorrhizal, it might be of ecological importance that one genotype reduces its indirect defence under these conditions, whereas the other one exhibits increased VOC emission. Whether this trend extends to other induced defences as well would be interesting to know. For example, mycorrhizal Vicia faba plants have been shown to produce fewer extrafloral nectaries than conspecifics without fungal symbionts (Laird and Addicott 2007). In addition, the qualitative differences between the cultivars regarding the composition of defensive compounds might also influence the resistance to diverse stressors. For example, linalool has been shown to act as repellent of aphids (Aharoni et al. 2003) and thus might contribute to enhanced resistance of cv. Paraggio to these herbivores.
The results are in line with previous findings, namely that mycorrhization influences the content of secondary metabolites in aboveground plant parts, and that these changes depend on the genetic background of the plant (Khaosaad et al. 2006). This offers a possible explanation for the high variability of plants’ responses to herbivore attack. Moreover, the fungal and herbivore species involved in the interaction also influence the outcome (Goverde et al. 2000; Gange et al. 2002, 2003; Bennett and Bever 2007). To what degree the plant species (or the plant cultivars) control these interplays is, however, only poorly understood.
To interpret the observed effects, two contradictory explanations might be taken into consideration: (a) AM fungi basically have to evade the plant’s defence responses in order to successfully colonise the roots. It is assumed that symbionts are able to suppress the plant’s defence mechanisms (Dumas-Gaudot et al. 2000), which could in part explain why induced defences are reduced in cv. Jemalong A17. (b) It is considered as a basic fact that mycorrhization increases the plant’s resistance to diverse stressors (Hause and Fester 2005). Therefore, in the case of cv. Paraggio, the slightly enhanced emission of certain VOCs may be a symptom of increased resistance or priming by AM fungi.
Changes in volatile patterns, be they increased or decreased emissions, might be causes for changed behaviour of plant mutualists. Most of the changes detected were, however, so small that their influence on such interactions seems improbable. Only the amounts of the two unidentified compounds (RI 1112 and RI 1038) varied to a large extent, and they were present in abundance that may lend support to their putative physiological and ecological roles. In order to carry out any investigation on that topic, the identification of those compounds is mandatory. But our knowledge about the importance of trace compounds is limited. Chemical detection limits do not necessarily transport crucial information about biological importance, as they do not take into account the sensitivity of biological perception systems.
Although the differences observed in volatile emission are only marginal, classification is clearly possible. This theoretical consideration is certainly of interest, demonstrating that the VOCs emitted by M. truncatula are characteristic enough to allow discrimination of different stimuli, even if the influences (on aerial plant parts) seem to be only minor. With regard to practical applicability, the problem gets more complicated. All experiments were conducted under highly controlled conditions. As VOCs of defined populations of plants were analysed, no problems with normalisation were encountered. Because the culture conditions were kept as constant as possible, variation due to the abiotic environment and biotic stresses can be assumed to be negligible. Finally, differences in the genetic background were comparatively low or well-defined. In short, any influence that could alter the plant’s emission patterns was reduced as much as possible. It is easily imaginable that the parameters listed above, and probably many more, severely impede successful discrimination of VOC blends in the natural environment. Regarding the statistical methods used, further tests still could improve the performance of classification. Though the results gained with LDA are quite satisfactory, other methods that are more robust with regard to multicollinearity and do not rely on linear correlations (e.g. neural networks, learning vector machines, etc.) could be expected to perform better. Indeed, several methods have been compared for other problems, such as classification of aromas that bear some similarity from the statistical point of view (Baroni et al. 2006; Gonzalez-Arjona et al. 2006). Still, the data presented may put the specificity and plasticity of induced volatile emission in a new perspective. Insight into multiple interactions seems to be substantially hindered by the fact that every organism involved has a drastic influence on the outcome. Thus, general effects are probably difficult to find, as the data available to date indicate that even the slightest variation in one of the partners of any interaction considerably changes the overall consequences.
References
Adams RP (2001) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured, Carol Stream
Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel W-J, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15:2866–2884
Akiyama K, Hayashi H (2002) Arbuscular mycorrhizal fungus-promoted accumulation of two new triterpenoids in cucumber roots. Biosci Biotechnol Biochem 66:762–769
Baroni MV, Nores ML, Diaz MDP, Chiabrando GA, Fassano JP, Costa C, Wunderlin DA (2006) Determination of volatile organic compound patterns characteristic of five unifloral honey by solid-phase microextraction-gas chromatography–mass spectrometry coupled to chemometrics. J Agric Food Chem 54:7235–7241
Bennett AE, Bever JD (2007) Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 88:210–218
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16:485–494
Currie AF, Murray PJ, Gange AC (2006) Root herbivory by Tipula paludosa larvae increases colonization of Agrostis capillaris by arbuscular mycorrhizal fungi. Soil Biol Biochem 38:1994–1997
Degen T, Dillmann C, Marion-Poll F, Turlings TCJ (2004) High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol 135:1928–1938
Donath J, Boland W (1995) Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry 39:785–790
Dumas-Gaudot E, Gollote A, Cordier C, Gianinazzi S, Gianinazzi-Pearson V (2000) Modulation of host defence systems. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology and function. Kluwer, Dortrecht, pp 173–200
Fester T, Hause B, Schmidt D, Halfmann K, Schmidt J, Wray V, Hause G, Strack D (2002) Occurrence and localization of apocarotenoids in arbuscular mycorrhizal plant roots. Plant Cell Physiol 43:256–265
Gange AC, Brown VK, Aplin DM (2003) Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecol Lett 6:1051–1055
Gange AC, Stagg PG, Ward LK (2002) Arbuscular mycorrhizal fungi affect phytophagous insect specialism. Ecol Lett 5:11–15
Gange AC, West HM (1994) Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol 128:79–87
Gehring C, Bennett A (2009) Mycorrhizal fungal–plant–insect interactions: the importance of a community approach. Environ Entomol 38:93–102
Gehring CA, Whitham TG (2002) Mycorrhizae–herbivore interactions: population and community consequences. In: Van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology, vol. 157. Springer, Berlin, pp 295–320
Gonzalez-Arjona D, Lopez-Perez G, Gonzalez-Gallero V, Gonzalez AG (2006) Supervised pattern recognition procedures for discrimination of whiskeys from gas chromatography/mass spectrometry congener analysis. J Agric Food Chem 54:1982–1989
Gouinguené S, Degen T, Turlings TCJ (2001) Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11:9–16
Goverde M, van der Heijden MGA, Wiemken A, Sanders IR, Erhardt A (2000) Arbuscular mycorrhizal fungi influence life history traits of a lepidopteran herbivore. Oecologia 125:362–369
Grandmaison J, Olah GM, Van Calsteren M-R, Furlan V (1993) Characterization and localization of plant phenolics likely involved in the pathogen resistance expressed by endomycorrhizal roots. Mycorrhiza 3:155–164
Guerrieri E, Lingua G, Digilio MC, Massa N, Berta G (2004) Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol Entomol 29:753–756
Harrison MJ, Dixon RA (1993) Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular–arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol Plant Microbe Interact 6:643–654
Hause B, Fester T (2005) Molecular and cell biology of arbuscular mycorrhizal symbiosis. Planta 221:184–196
Hause B, Mrosk C, Isayenkov S, Strack D (2007) Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 68:101–110
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition, 2nd edn. Commonwealth Agricultural Bureaux, Farnham Royal, pp 187–237
Hochmuth DH (2004) MassFinder 3. Hamburg. http://www.massfinder.com
Hoffmann D, Vierheilig H, Riegler P, Schausberger P (2009) Arbuscular mycorrhizal symbiosis increases host plant acceptance and population growth rates of the two-spotted spider mite Tetranychus urticae. Oecologia 158:663–671
Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J (2006) Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamicaeae). Mycorrhiza 16:443–446
Kruskal JB (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129
Laird RA, Addicott JF (2007) Arbuscular mycorrhizal fungi reduce the construction of extrafloral nectaries in Vicia faba. Oecologia 152:541–551
Larose G, Chênevert R, Moutoglis P, Gagné S, Piché Y, Vierheilig H (2002) Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J Plant Physiol 159:1329–1339
Leitner M, Boland W, Mithöfer A (2005) Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol 167:597–606
Leitner M, Kaiser R, Rasmussen MO, Driguez H, Boland W, Mithöfer A (2008) Microbial oligosaccharides differentially induce volatiles and signalling components in Medicago truncatula. Phytochemistry 69:2029–2040
Linstrom PJ, Mallard WG (2005) NIST chemistry WebBook, NIST standard reference database number 69. Gaithersburg: National Institute of Standards and Technology. http://webbook.nist.gov
Loughrin JH, Manukian A, Heath RR, Tumlinson JH (1995) Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol 21:1217–1227
Maier W, Peipp H, Schmidt J, Wray V, Strack D (1995) Levels of a terpenoid glycoside (blumenin) and cell wall-bound phenolics in some cereal mycorrhizas. Plant Physiol 109:465–470
Mardia KV, Kent JT, Bibby JM (1979) Multivariate analysis. Academic, New York
McLachlan GJ (1992) Discriminant analysis and statistical pattern recognition. Wiley, New York
Minchin PR (1987) An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 69:89–107
Mithöfer A, Boland W, Maffei ME (2009) Chemical ecology of plant–insect interactions. In: Parker J (ed) Annual plant reviews: molecular aspects of plant disease resistance. Wiley-Blackwell, Chichester, pp 261–291
Nair RM, Howie JH, Delalande M (2006) Medicago truncatula cultivars. In: The Medicago truncatula handbook. http://www.noble.org/MedicagoHandbook/
Oksanen J, Kindt R, Legendre P, O'Hara RB (2006) vegan: community ecology package. R package version 1.8-3
Peipp H, Maier W, Schmidt J, Wray V, Strack D (1997) Arbuscular mycorrhizal fungus-induced changes in the accumulation of secondary compounds in barley roots. Phytochemistry 44:581–587
Phillips JM, Hayman DS (1970) Improved procedures for cleaning roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–162
Rapparini F, Llusià J, Penuelas J (2008) Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua L. Plant Biol 10:108–122
Royston P (1982) Algorithm AS 181: the W test for normality. Appl Stat 31:176–180
Schliemann W, Ammer C, Strack D (2008) Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 69:112–146
Strack D, Fester T, Hause B, Schliemann W, Walter MH (2003) Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol 29:1955–1979
Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2:1–6
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
Weihs C, Ligges U, Luebke K, Raabe N (2005) klaR analyzing German business cycles. In: Baier D, Decker R, Schmidt-Thieme L (eds) Data analysis and decision support. Springer, Berlin, pp 335–343
Wolfe BE, Husband BC, Klironomos JN (2005) Effects of a belowground mutualism on an aboveground mutualism. Ecol Lett 8:218–223
Acknowledgements
We like to thank Dr. J.M. Prosperi (INRA-SGAP, Montpellier, France) for providing M. truncatula seeds, Dr. R. Kaiser (Givaudan Company, Dübendorf, Switzerland) and Dr. S.H. von Reuß (University of Hamburg, Hamburg, Germany) for terpenoid standards, and the Max-Planck-Gesellschaft for financial support. We thank Angelika Berg for insect rearing.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
(JPG 346 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Leitner, M., Kaiser, R., Hause, B. et al. Does mycorrhization influence herbivore-induced volatile emission in Medicago truncatula?. Mycorrhiza 20, 89–101 (2010). https://doi.org/10.1007/s00572-009-0264-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-009-0264-z