Abstract
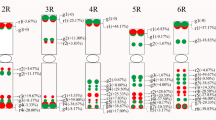
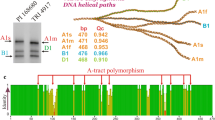
Photoperiod response in wheat is determined to a large extent by the homoeologous series of Photoperiod 1 (Ppd1) genes. In this study, Ppd-A1 genomic sequences from the 5′ UTR and promoter region were analysed in 104 accessions of six tetraploid wheat species (Triticum dicoccoides, T. dicoccum, T. turgidum, T. polonicum, T. carthlicum, T. durum) and 102 accessions of six hexaploid wheat species (T. aestivum, T. compactum, T. sphaerococcum, T. spelta, T. macha, T. vavilovii). This data was supplemented with in silico analysis of publicly available sequences from 46 to 193 accessions of diploid and tetraploid wheat, respectively. Analysis of a region of the Ppd-A1 promoter identified thirteen haplotypes, which were divided in two haplogroups. Distribution of the Ppd-A1 haplogroups and haplotypes in wheat species, and their geographical distributions were analysed. Polymerase chain reaction combined with a heteroduplex mobility assay was subsequently used to efficiently discriminate between Ppd-A1 alleles, allowing identification of the Ppd-A1b haplotypes and haplogroups. The causes of anomalous migration of Ppd-A1 heteroduplexes in gels were found to be the localization of mismatches relative to the center of fragment, the cumulative effect of neighbouring polymorphic sites, and the location of mismatches within A/T-tracts. Analysis of the Ppd-A1 5′ UTR in hexaploid wheat revealed a novel mutation within the “photoperiod critical” region in a subset of T. compactum accessions. This putative photoperiod insensitive allele (designated Ppd-A1a.4) includes a 684 bp deletion which spans region in common with deletions previously identified in other photoperiod insensitive Ppd1 alleles.







Similar content being viewed by others
References
Andersen JR, Lübberstedt T (2003) Functional markers in plants. Trends Plant Sci 8(11):554–560
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16(1):37–48
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115(5):721–733
Bentley AR, Turner AS, Gosman N, Leigh FJ, Maccaferri M, Dreisigacker S, Greenland A, Laurie DA (2011) Frequency of photoperiod-insensitive Ppd-A1a alleles in tetraploid, hexaploid and synthetic hexaploid wheat germplasm. Plant Breed 130(1):10–15
Bentley AR, Jensen EF, Mackay IJ, Hönicka H, Fladung M, Hori K, Yano M, Mullet JE, Armstead IP, Hayes C, Thorogood D, Lovatt A, Morris R, Pullen N, Mutasa-Göttgens E, Cockram J (2013) Flowering time. In: Cole C (ed) Genomics and breeding for climate-resilient crops 2. Springer, Berlin, pp 1–67
Bowyer J, Verrills N, Gillings MR, Holmes AJ (2000) Heteroduplex mobility assay as a tool for predicting phylogenetic affiliation of environmental ribosomal RNA clones. J Microbiol Methods 41(2):155–160
Budowle B, Chakraborty R, Giusti AM, Eisenberg AJ, Allen RC (1991) Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet 48(1):137–144
Campoli C, Drosse B, Searle I, Coupland G, von Korff M (2012) Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J 69(5):868–880
Cockram J, Jones H, Leigh FJ, O’Sullivan D, Powell W, Laurie DA, Greenland AJ (2007a) Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. J Exp Bot 58(6):1231–1244
Cockram J, Mackay IJ, O’Sullivan DM (2007b) The role of double-stranded break repair in the creation of phenotypic diversity at cereal VRN1 loci. Genetics 177(4):2535–2539
Cockram J, Thiel T, Steuernagel B, Stein N, Taudien S, Bailey PC, O’Sullivan DM (2012) Genome dynamics explain the evolution of flowering time CCT domain gene families in the Poaceae. PLoS One 7:e45307
Delwart EL, Shpaer EG, Louwagie J, McCutchan FE, Grez M, Rübsamen-Waigmann H, Mullins JI (1993) Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262(5137):1257–1261
Diaz A, Zikhali M, Turner A, Isaac P, Laurie D (2012) Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One 7(3):e33234
Diekmann S (1989) The migration anomaly of DNA fragments in polyacrylamide gels allows the detection of small sequence-specific DNA structure variations. Electrophoresis 10(5–6):354–359
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Ellerton S (1939) The origin and geographical distribution of Triticum sphaerococcum prec. and its cytogenetical behaviour in crosses with T. vulgare Vill. J Genet 38:307–324
Feldman M, Levy AA (2012) Genome evolution due to allopolyploidization in wheat. Genetics 192(3):763–774
Ganguly A, Rock MJ, Prockop DJ (1993) Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA 90(21):10325–10329
Golovnina KA, Glushkov SA, Blinov AG, Mayorov VI, Adkison LR, Goncharov NP (2007) Molecular phylogeny of the genus Triticum L. Plant Syst Evol 264(3–4):195–216
Guo Z, Song Y, Zhou R, Ren Z, Jia J (2010) Discovery, evaluation and distribution of haplotypes of the Ppd-D1 gene. New Phytol 185:841–851
Gusfield D (1997) Algorithms on strings, trees, and sequences: computer science and computational biology. Cambridge University Press, Cambridge
Haudry A, Cenci A, Ravel C, Bataillon T, Brunel D, Poncet C, Hochu I, Poirier S, Santoni S, Glémin S, David J (2007) Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol Biol Evol 24(7):1506–1517
Huang T, Yeh Y, Tzeng DD (2010) Heteroduplex mobility assay for identification and phylogenetic analysis of anthracnose fungi. J Phytopathol 158(1):46–55
Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R (2007) Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinform 8(1):460–466
Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309(5732):293–297
Johnson BL, Dhaliwal HS (1976) Reproductive isolation of Triticum boeoticum and Triticum urartu and the origin of the tetraploid wheat. Am J Bot 63(8):1088–1094
Jones H, Leigh FJ, Mackay I, Bower MA, Smith LMJ, Charles MP, Jones G, Jones MK, Brown TA, Powell W (2008) Population-based resequencing reveals that the flowering time adaptation of cultivated barley originated east of the Fertile Crescent. Mol Biol Evol 25:2211–2219
Jones H, Civan P, Cockram J, Leigh FJ, Smith LMJ, Jones MK, Charles MP, Molina-Cano J-L, Powell W, Jones G, Brown TA (2011) Evolutionary history of barley cultivation in Europe revealed by genetic analysis of extant landraces. BMC Evolut Biol 11:e320
Kato K, Yokoyama H (1992) Geographical variation in heading characters among wheat landraces, Triticum aestivum L., and its implication for their adaptability. Theor Appl Genet 84(3–4):259–265
Kilian B, Özkan H, Pozzi C, Salamini F (2009) Domestication of the Triticeae in the Fertile Crescent. In: Muehlbauer GJ (ed) Genetics and genomics of the Triticeae. Springer, New York, pp 81–119
Koo HS, Wu HM, Crothers DM (1986) DNA bending at adenine-thymine tracts. Nature 320(6062):501–506
Lavery R, Moakher M, Maddocks JH, Petkeviciute D, Zakrzewska K (2009) Conformational analysis of nucleic acids revisited: curves+. Nucleic Acids Res 37(17):5917–5929
Law CN, Worland AJ (1997) Genetic analysis of some flowering time and adaptive traits in wheat. New Phytol 137:19–28
Makarenkov V, Kevorkov D, Legendre P (2006) Phylogenetic network construction approaches. Appl Mycol Biotechnol 6:61–97
Marini JC, Levene SD, Crothers DM, Englund PT (1982) Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci USA 79(24):7664–7668
Matsuoka Y (2011) Evolution of polyploid Triticum wheats under cultivation: the role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol 52(5):750–764
Mizuno T, Nakamichi N (2005) Pseudo-response regulators (PRRs) or true oscillator components (TOCs). Plant Cell Physiol 46(5):677–685
Mount DW (2008) Choosing a method for phylogenetic prediction. CSH Protoc pdb.ip49
Nakamichi N, Kita M, Niinuma K, Ito S, Yamashino T, Mizoguchi T, Mizuno T (2007) Arabidopsis clock-associated pseudo-response regulators PRR9, PRR7 and PRR5 coordinately and positively regulate flowering time through the canonical CONSTANS-dependent photoperiodic pathway. Plant Cell Physiol 48(6):822–832
Nishida H, Yoshida T, Kawakami K, Fujita M, Long B, Akashi Y, Laurie DA, Kato K (2013) Structural variation in the 5′ upstream region of photoperiod-insensitive alleles Ppd-A1a and Ppd-B1a identified in hexaploid wheat (Triticum aestivum L.), and their effect on heading time. Mol Breed 31(1):27–37
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Polzin T, Daneschmand SV (2003) On Steiner trees and minimum spanning trees in hypergraphs. Oper Res Lett 31(1):12–20
Puchta H (2005) The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J Exp Bot 56(409):1–14
Scarth R, Law CN (1984) The control of day-length response in wheat by the group 2 chromosomes. Z Pflanzenzücht 92:140–150
Shaw LM, Turner AS, Laurie DA (2012) The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). Plant J 71(1):71–84
Stellwagen NC (2009) Electrophoresis of DNA in agarose gels, polyacrylamide gels and in free solution. Electrophoresis 30(1):188–195
Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410(6832):1116–1120
Takenaka S, Kawahara T (2012) Evolution and dispersal of emmer wheat (Triticum sp.) from novel haplotypes of Ppd-1 (photoperiod response) genes and their surrounding DNA sequences. Theor Appl Genet 125(5):999–1014
Takenaka S, Kawahara T (2013) Evolution of tetraploid wheat based on variations in 5′ UTR regions of Ppd-A1: evidence of gene flow between emmer and timopheevi wheat. Genet Resour Crop Evol 60(7):2143–2155
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310(5750):1031–1034
Upchurch DA, Shankarappa R, Mullins JI (2000) Position and degree of mismatches and the mobility of DNA heteroduplexes. Nucleic Acids Res 28(12):E69
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303(5660):1003–1006
Wilhelm EP, Turner AS, Laurie DA (2009) Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor Appl Genet 118(2):285–294
Worland AJ, Börner A, Korzun V, Li WM, Petrovic S, Sayers EJ (1998) The influence of photoperiod genes on the adaptability of European winter wheats. Euphytica 100(1–3):385–394
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muterko, A., Kalendar, R., Cockram, J. et al. Discovery, evaluation and distribution of haplotypes and new alleles of the Photoperiod-A1 gene in wheat. Plant Mol Biol 88, 149–164 (2015). https://doi.org/10.1007/s11103-015-0313-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0313-2