Abstract
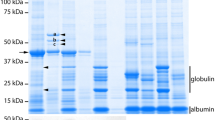
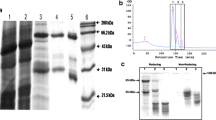
Antibodies and antibody derived fragments are excellent tools for the detection and purification of proteins. However, only few antibodies targeting Arabidopsis seed proteins are currently available. Here, we evaluate the process to make antibody libraries against crude protein extracts and more particularly to generate a VHH phage library against native Arabidopsis thaliana seed proteins. After immunising a dromedary with a crude Arabidopsis seed extract, we cloned the single-domain antigen-binding fragments from their heavy-chain only antibodies in a phage display vector and selected nanobodies (VHHs) against native Arabidopsis seed proteins. For 16 VHHs, the corresponding antigens were identified by affinity purification and MS/MS analysis. They were shown to bind the major Arabidopsis seed storage proteins albumin and globulin (14 to albumin and 2 to globulin). All 16 VHHs were suitable primary reagents for the detection of the Arabidopsis seed storage proteins by ELISA. Furthermore, several of the anti-albumin VHHs were used successfully for storage protein localisation via electron microscopy. The easy cloning, selection and production, together with the demonstrated functionality and applicability, strongly suggest that the VHH antibody format will play a more prominent role in future protein research, in particular for the study of native proteins.





Similar content being viewed by others
References
Abbady AQ, Al-Mariri A, Zarkawi M, Al-Assad A, Muyldermans S (2011) Evaluation of a nanobody phage display library constructed from a Brucella-immunised camel. Vet Immunol Immunopathol 142(1–2):49–56. doi:10.1016/j.vetimm.2011.04.004
Abbady AQ, Al-Daoude A, Al-Mariri A, Zarkawi M, Muyldermans S (2012) Chaperonin GroEL a Brucella immunodominant antigen identified using Nanobody and MALDI-TOF-MS technologies. Vet Immunol Immunopathol 146(3–4):254–263. doi:10.1016/j.vetimm.2012.01.015
Adachi M, Kanamori J, Masuda T, Yagasaki K, Kitamura K, Mikami B, Utsumi S (2003) Crystal structure of soybean 11S globulin: glycinin A3B4 homohexamer. Proc Natl Acad Sci USA 100(12):7395–7400. doi:10.1073/pnas.0832158100
Arbabi-Ghahroudi M, Tanha J, MacKenzie R (2005) Prokaryotic expression of antibodies. Cancer Metastasis Rev 24(4):501–519. doi:10.1007/s10555-005-6193-1
Baens M, Noels H, Broeckx V, Hagens S, Fevery S, Billiau AD, Vankelecom H, Marynen P (2006) The dark side of EGFP: defective polyubiquitination. PLoS One 1(1):e54. doi:10.1371/journal.pone.0000054
Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S (2008) Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320(5878):938–941. doi:10.1126/science.1157956
Bernal AJ, Willats WGT (2004) Plant science in the age of phage. Trends Plant Sci 9(10):465–468. doi:10.1016/j.tplants.2004.08.004
Claeys M, Vanhecke D, Couvreur M, Tytgat T, Coomans A, Borgonie G (2004) High-pressure freezing and freeze substitution of gravid Caenorhabditis elegans (Nematoda: Rhabditida) for transmission electron microscopy. Nematology 6(3):319–327. doi:10.1163/1568541042360564
De Buck S, Virdi V, De Meyer T, De Wilde K, Piron R, Nolf J, Van Lerberge E, De Paepe A, Depicker A (2012) Production of camel-like antibodies in plants. Methods Mol Biol 911:305–324
De Clercq A, Vandewiele M, De Rycke R, Van Damme J, Van Montagu M, Krebbers E, Vandekerckhove J (1990) Expression and processing of an Arabidopsis 2S albumin in transgenic tobacco. Plant Physiol 92(4):899–907. doi:10.1104/pp.92.4.899
De Genst E, Silence K, Decanniere K, Conrath K, Loris R, Kinne R, Muyldermans S, Wyns L (2006) Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci USA 103(12):4586–4591. doi:10.1073/pnas.0505379103
Deckers N, Saerens D, Kanobana K, Conrath K, Victor B, Wernery U, Vercruysse J, Muyldermans S, Dorny P (2009) Nanobodies, a promising tool for species-specific diagnosis of Taenia solium cysticercosis. Int J Parasit 39(5):625–633. doi:10.1016/j.ijpara.2008.10.012
Dübel S, Stoevesandt O, Taussig MJ, Hust M (2010) Generating recombinant antibodies to the complete human proteome. Trends Biotechnol 28(7):333–339. doi:10.1016/j.tibtech.2010.05.001
Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, Muyldermans S, Wyns L, Matagne A (2002) Single-domain antibody fragments with high conformational stability. Protein Sci 11(3):500–515. doi:10.1110/ps.34602
Eeckhout D, De Clercq A, Van De Slijke E, Van Leene J, Stals H, Casteels P, Persiau G, Vercammen D, Van Breusegem F, Zabeau M, Inzé D, Jespers L, Depicker A, De Jaeger G (2004) A technology platform for the fast production of monoclonal recombinant antibodies against plant proteins and peptides. J Immunol Methods 294(1–2):181–187. doi:10.1016/j.jim.2004.08.006
Freydank A-C, Brandt W, Dräger B (2008) Protein structure modeling indicates hexahistidine-tag interference with enzyme activity. Proteins 72(1):173–183. doi:10.1002/prot.21905
Fujiwara T, Nambara E, Yamagishi K, Goto DB, Naito S (2002) Storage proteins. In: Somerville CR, Meyerowitz EM (eds) The arabidopsis book, vol 1. American Society of Plant Physiologists, Rockville, pp 1–12. doi:10.1199/tab.0020
Ghassabeh GH, Saerens D, Muyldermans S (2010) Isolation of antigen-specific nanobodies. In: Kontermann R, Dübel S (eds) Antibody engineering, vol 2. Springer, Berlin, pp 251–266
Goossens A, Dillen W, De Clercq J, Van Montagu M, Angenon G (1999) The arcelin-5 gene of Phaseolus vulgaris directs high seed-specific expression in transgenic Phaseolus acutifolius and Arabidopsis plants. Plant Physiol 120(4):1095–1104
Gorlani A, de Haard H, Verrips T (2012) Expression of VHHs in Saccharomyces cerevisiae. Methods Mol Biol 911:277–286. doi:10.1007/978-1-61779-968-6_17
Groot AJ, El Khattabi M, Sachs N, van der Groep P, van der Wall E, van Diest PJ, Sonnenberg A, Verrips CT, Vooijs M (2009) Reverse proteomic antibody screening identifies anti adhesive VHH targeting VLA-3. Mol Immunol 46(10):2022–2028. doi:10.1016/j.molimm.2009.03.002
Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R (1993) Naturally occurring antibodies devoid of light chains. Nature 363(6428):446–448. doi:10.1038/363446a0
Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10(5):825–836
Harmsen MM, De Haard HJ (2007) Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 77(1):13–22. doi:10.1007/s00253-007-1142-2
Herman EM, Larkins BA (1999) Protein storage bodies and vacuoles. Plant Cell 11(4):601–613
Higashi Y, Hirai MY, Fujiwara T, Naito S, Noji M, Saito K (2006) Proteomic and transcriptomic analysis of Arabidopsis seeds: molecular evidence for successive processing of seed proteins and its implication in the stress response to sulfur nutrition. Plant J 48(4):557–571. doi:10.1111/j.1365-313X.2006.02900.x
Hmila I, Abdallah RBA-B, Saerens D, Benlasfar Z, Conrath K, Ayeb ME, Muyldermans S, Bouhaouala-Zahar B (2008) VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Mol Immunol 45(14):3847–3856. doi:10.1016/j.molimm.2008.04.011
Hu W-G, Yin J, Jager S, Wong C, Fulton C, Rayner GA, Aw C, Fisher GR, Dai X, Nagata LP (2008) A novel approach to development of monoclonal antibodies using native antigen for immunization and recombinant antigen for screening. Hybridoma 27(4):307–311. doi:10.1089/hyb.2008.0011
Jenkins JA, Griffiths-Jones S, Shewry PR, Breiteneder H, Mills ENC (2005) Structural relatedness of plant food allergens with specific reference to cross-reactive allergens: an in silico analysis. J Allergy Clin Immunol 115(1):163–170. doi:10.1016/j.jaci.2004.10.026
Jensen BC, Swigart PM, Simpson PC (2009) Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn-Schmiedeberg’s Arch Pharmacol 379(4):409–412. doi:10.1007/s00210-008-0368-6
Krebbers E, Herdies L, De Clercq A, Seurinck J, Leemans J, Van Damme J, Segura M, Gheysen G, Van Montagu M, Vandekerckhove J (1988) Determination of the processing sites of an Arabidopsis 2S albumin and characterization of the complete gene family. Plant Physiol 87(4):859–866. doi:10.1104/pp.87.4.859
Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Hölzer W, De Genst E, Wyns L, Muyldermans S (1998) Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J 17(13):3512–3520. doi:10.1093/emboj/17.13.3512
Leduc C, Si S, Gautier J, Soto-Ribeiro M, Wehrle-Haller B, Gautreau A, Giannone G, Cognet L, Lounis B (2013) A highly specific gold nanoprobe for live-cell single-molecule imaging. Nano Lett 13(4):1489–1494. doi:10.1021/nl304561g
Moutel S, El Marjou A, Vielemeyer O, Nizak C, Benaroch P, Dübel S, Perez F (2009) A multi-Fc-species system for recombinant antibody production. BMC Biotechnol 9:14. doi:10.1186/1472-6750-9-14
Müntz K (1998) Deposition of storage proteins. Plant Mol Biol 38(1–2):77–99. doi:10.1007/978-94-011-5298-3_4
Muyldermans S (2013) Nanobodies: natural single-domain antibodies. Annu Rev Biochem. doi:10.1146/annurev-biochem-063011-092449
Nilsson P, Paavilainen L, Larsson K, Ödling J, Sundberg M, Andersson A-C, Kampf C, Persson A, Al-Khalili Szigyarto C, Ottosson J, Björling E, Hober S, Wernérus H, Wester K, Pontén F, Uhlen M (2005) Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics 5(17):4327–4337. doi:10.1002/pmic.200500072
Pang PP, Pruitt RE, Meyerowitz EM (1988) Molecular cloning, genomic organization, expression and evolution of 12S seed storage protein genes of Arabidopsis thaliana. Plant Mol Biol 11(6):805–820. doi:10.1007/BF00019521
Parmley SF, Smith GP (1988) Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 73(2):305–318. doi:10.1016/0378-1119(88)90495-7
Ries J, Kaplan C, Platonova E, Eghlidi H, Ewers H (2012) A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nat Methods 9(6):582–584. doi:10.1038/nmeth.1991
Rothbauer U, Zolghadr K, Tillib S, Nowak D, Schermelleh L, Gahl A, Backmann N, Conrath K, Muyldermans S, Cardoso MC, Leonhardt H (2006) Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods 3(11):887–889. doi:10.1038/nmeth953
Rothbauer U, Zolghadr K, Muyldermans S, Schepers A, Cardoso MC, Leonhardt H (2008) A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Proteomics 7(2):282–289. doi:10.1074/mcp.M700342-MCP200
Saerens D, Stijlemans B, Baral TN, Nguyen Thi GT, Wernery U, Magez S, De Baetselier P, Muyldermans S, Conrath K (2008) Parallel selection of multiple anti-infectome Nanobodies without access to purified antigens. J Immunol Methods 329(1–2):138–150. doi:10.1016/j.jim.2007.10.005
Sjodahl S, Rodin J, Rask L (1991) Characterization of the 12 s globulin complex of brassica-napus: evolutionary relationship to other 11–12 s storage globulins. Eur J Biochem 196(3):617–621
Snapp EL (2009) Fluorescent proteins: a cell biologist’s user guide. Trends Cell Biol 19(11):649–655. doi:10.1016/j.tcb.2009.08.002
Stoevesandt O, Taussig MJ (2012) European and international collaboration in affinity proteomics. New Biotechnol 29(5):511–514. doi:10.1016/j.nbt.2012.05.003
Tandang MRG, Atsuta N, Maruyama N, Adachi M, Utsumi S (2005) Evaluation of the solubility and emulsifying property of soybean proglycinin and rapeseed procruciferin in relation to structure modified by protein engineering. J Agric Food Chem 53(22):8736–8744. doi:10.1021/Jf050871y
van der Klei H, Van Damme J, Casteels P, Krebbers E (1993) A fifth 2S albumin isoform is present in Arabidopsis thaliana. Plant Physiol 101(4):1415–1416
Van Leene J, Hollunder J, Eeckhout D, Persiau G, Van De Slijke E, Stals H, Van Isterdael G, Verkest A, Neirynck S, Buffel Y, De Bodt S, Maere S, Laukens K, Pharazyn A, Ferreira PCG, Eloy N, Renne C, Meyer C, Faure J-D, Steinbrenner J, Beynon J, Larkin JC, Van de Peer Y, Hilson P, Kuiper M, De Veylder L, Van Onckelen H, Inzé D, Witters E, De Jaeger G (2010) Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol 6:397. doi:10.1038/msb.2010.53
Vanhecke D, Graber W, Studer D (2008) Close-to-native ultrastructural preservation by high pressure freezing. Methods Cell Biol 88:151–164. doi:10.1016/S0091-679X(08)00409-3
Vanlandschoot P, Stortelers C, Beirnaert E, Ibañez LI, Schepens B, Depla E, Saelens X (2011) Nanobodies®: new ammunition to battle viruses. Antiviral Res 92(3):389–407. doi:10.1016/j.antiviral.2011.09.002
Yewdell JW, Lacsina JR, Rechsteiner MC, Nicchitta CV (2011) Out with the old, in with the new? Comparing methods for measuring protein degradation. Cell Biol Int 35(5):457–462. doi:10.1042/CBI20110055
Zhang J, Tanha J, Hirama T, Khieu NH, To R, Tong-Sevinc H, Stone E, Brisson J-R, MacKenzie CR (2004) Pentamerization of single-domain antibodies from phage libraries: a novel strategy for the rapid generation of high-avidity antibody reagents. J Mol Biol 335(1):49–56. doi:10.1016/j.jmb.2003.09.034
Zhu X, Wang L, Liu R, Flutter B, Li S, Ding J, Tao H, Liu C, Sun M, Gao B (2010) COMBODY: one-domain antibody multimer with improved avidity. Immunol Cell Biol 88(6):667–675. doi:10.1038/icb.2010.21
Acknowledgments
The authors thank Geert De Jaeger and his research group for advice and help, Gholamreza Hassanzadeh Ghassabeh for helpful discussions and for providing the pHEN6c-GBP vector, Jonah Nolf and Gijs De Cort for technical assistance and Annick Bleys for help in preparing the manuscript. T.D.M. is indebted to the Agency for Innovation by Science and Technology (IWT) for a predoctoral fellowship.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Meyer, T., Eeckhout, D., De Rycke, R. et al. Generation of VHH antibodies against the Arabidopsis thaliana seed storage proteins. Plant Mol Biol 84, 83–93 (2014). https://doi.org/10.1007/s11103-013-0118-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-013-0118-0