Abstract
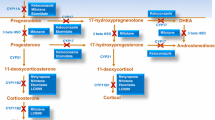
The expression of dopamine receptor subtypes has been reported in corticotroph adenomas, and this finding support the possibility for medical treatment of Cushing’s disease (CD) with dopamine agonists when conventional treatment has failed. The aim of this study was to evaluate the effectiveness of cabergoline (at doses of up 3 mg/week), alone or combined with relatively low doses of ketoconazole (up to 400 mg/day), in 12 patients with CD unsuccessfully treated by transsphenoidal surgery. After 6 months of cabergoline therapy, normalization of 24 h urinary free cortisol (UFC) levels occurred in three patients (25%) at doses ranging from 2–3 mg/week, whereas reductions ranging from 15.0 to 48.4% were found in the remaining. The addition of ketonocazole to the nine patients without an adequate response to cabergoline was able to normalize UFC excretion in six patients (66.7%) at doses of 200 mg/day (three patients), 300 mg/day (two patients) and 400 mg/day (one patient). In the remaining patients UFC levels did not normalize but a significant reduction ranging from to 44.4 to 51.7% was achieved. In two of the six responsive patients to combination therapy, the weekly dose of cabergoline could be later reduced from 3 to 2 mg. Our findings demonstrated that cabergoline monotherapy was able to reverse hypercortisolism in 25% of patients with CD unsuccessfully treated by surgery. Moreover, the addition of relatively low doses of ketoconazole led to normalization of UFC in about two-thirds of patients not achieving a full response to cabergoline.


Similar content being viewed by others
References
Pivonello R, De Martino MC, De Leo M et al (2008) Cushing’s syndrome. Endocrinol Metab Clin North Am 37:135–149
Nieman LK, Biller BM, Findling JW et al (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:1526–1540
Vilar L, Freitas MC, Faria M et al (2007) Pitfalls in the diagnosis of Cushing’s syndrome. Arq Brasil Endocrinol Metab 51:1207–1216
Biller BM, Grossman AB, Stewart PM et al (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Newell-Price J, Bertagna X, Grossman AB et al (2006) Cushing’s syndrome. Lancet 367:1605–1617
Vance ML (2009) Cushing’s disease: radiation therapy. Pituitary 12:11–14
Smith PW, Turza KC, Carter CO et al (2009) Bilateral adrenalectomy for refractory Cushing disease: a safe and definitive therapy. J Am Coll Surg 208:1059–1064
Dang CN, Trainer P (2007) Pharmacological management of Cushing’s syndrome: an update. Arq Bras Endocrinol Metabol 51:1339–1348
Moncet D, Morando DJ, Pitoia F et al (2007) Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing’s syndrome. Medicina (B Aires) 67:26–31
Sonino N, Boscaro M (1999) Medical therapy for Cushing’s disease. Endocrinol Metab Clin North Am 28:211–222
Lamberts SW, Klijn JG, De Quijada M et al (1980) The mechanism of the suppressive action of bromocriptine on adrenocorticotropin secretion in patients with Cushing’s disease and Nelson’s syndrome. J Clin Endocrinol Metab 51:307–311
Krieger DT, Amorosa L, Linick F (1975) Cyproheptadine-induced remission of Cushing’s disease. N Engl J Med 293:893–896
Colao A, Pivonello R, Tripodi FS et al (1997) Failure of long-term therapy with sodium valproate in Cushing’s disease. J Endocrinol Invest 20:387–392
Pivonello R, De Martino MC, Cappabianca P et al (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94:223–230
Pivonello R, Faggiano A, Di Salle F et al (1999) Complete remission of Nelson’s syndrome after 1-year treatment with cabergoline. J Endocrinol Invest 22:860–886
Casulari LA, Naves LA, Mello PA et al (2004) Nelson’s syndrome: complete remission with cabergoline but not with bromocriptine or cyproheptadine treatment. Horm Res 62:300–305
Boscaro M, Ludlam WH, Atkinson B et al (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94:115–122
Vilar L, Freitas MC, Naves LA et al (2008) The role of non-invasive dynamic tests in the diagnosis of Cushing’s syndrome. J Endocrinol Invest 31:1008–1013
Miller JW, Crapo L (1993) The medical treatment of Cushing’s syndrome. Endocr Rev 14:443–458
Lamberts SW, Birkenhager JC (1976) Bromocriptine in Nelson’s syndrome and Cushing’s disease. Lancet 2:811
Lamberts SW, Timmermans HA, De Jong FH, Birkenhager JC (1977) The role of dopaminergic depletion in the pathogenesis of Cushing’s disease and the possible consequences for medical therapy. Clin Endocrinol (Oxf) 7:185–193
Mercado-Asis LB, Yasuda K, Murayama M et al (1992) Beneficial effects of high daily dose bromocriptine treatment in Cushing’s disease. Endocrinol Jpn 39:385–395
Bevan JS, Webster J, Burke CW, Scanlon MF (1992) Dopamine agonists and pituitary tumor shrinkage. Endocr Rev 13:220–240
Petrossians P, Ronci N, Valdes-Socin H et al (2001) ACTH silent adenoma shrinking under cabergoline. Eur J Endocrinol 144:51–57
Miyoshi T, Otsuka F, Takeda M et al (2004) Effect of cabergoline treatment on Cushing’s disease caused by aberrant adrenocorticotropin-secreting macroadenoma. J Endocrinol Invest 27:1055–1059
Tsjoen G, Defeyter I, Van De Saffele J, Rubens R, Vandeweghe M (2002) Macroprolactinoma associated with Cushing’s disease, successfully treated with cabergoline. J Endocrinol Invest 25:172–175
Pivonello R, Ferone D, de Herder WW et al (2004) Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab 89:2452–2462
de Bruin C, Pereira AM, Feelders RA et al (2009) Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J Clin Endocrinol Metab 94:1118–1124
Godbout A, Manavela M, Danilowicz K et al. (2008) Long-term therapy with cabergoline in Cushing’s disease. Program of the 90th annual meeting of the endocrine society, San Francisco, CA, 2008 (p 2–130)
Colao A, Lombardi G, Annunziato L (2000) Cabergoline. Expert Opin Pharmacother 1:555–574
Lamberts SW, de Lange SA, Stefanko SZ (1982) Adrenocorticotropin-secreting pituitary adenomas originate from the anterior or the intermediate lobe in Cushing’s disease: differences in the regulation of hormone secretion. J Clin Endocrinol Metab 54:286–291
Croughs RJM, Koppeschaar HPF, Van’t Verlaat JW, McNicol AM (1989) Bromocriptine-responsive Cushing’s disease associated with anterior pituitary corticotroph hyperplasia or normal pituitary gland. J Clin Endocrinol Metab 68:495–498
Bricaire L, Brue T (2007) New medical treatments in Cushing’s disease. Ann Endocrinol (Paris) 68(Suppl 1):18–20
Colao A, Galderisi M, Di Sarno A et al (2008) Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocr Metab 93:3777–3784
Czepielewski MA, Rollin GAFS, Bruno OD, Vilar L (2009) Treatment of Cushing’s syndrome. In: Vilar L (ed) Clinical Endocrinology, 4th edn. Guanabara Koogan, Rio, pp 459–475
Colao A, Di Sarno A, Marzullo P et al (2000) New medical approaches in pituitary adenomas. Horm Res 53(Suppl 3):76–87
Pivonello R, De Leo M, De Martino MC et al. (2009) Effeectiveness and safety of combined therapy with low dose ketoconazole and cabergoline in patients with Cushing’s disease partially responsive to monotherapy with cabergoline. Program of the 11th international pituitary congress, Washington, DC, pp 36–40
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilar, L., Naves, L.A., Azevedo, M.F. et al. Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary 13, 123–129 (2010). https://doi.org/10.1007/s11102-009-0209-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-009-0209-8