Abstract
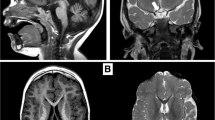
Septo-optic dysplasia (SOD) is a highly heterogeneous condition comprising a variable phenotype of optic nerve hypoplasia, midline forebrain abnormalities and pituitary hypoplasia with consequent endocrine deficits. The majority of cases are sporadic and several aetiologies including drug and alcohol abuse have been suggested to account for the pathogenesis of the condition. However, a number of familial cases have been described and the identification of mutations in the key developmental gene HESX1 in patients with SOD and associated phenotypes suggests that a genetic causation is likely in the more common sporadic cases of the condition. More recently, we have implicated duplications of SOX3 and mutations of both SOX2 and SOX3 in the aetiology of variants of SOD. As with other developmental disorders such as holoprosencephaly, the precise aetiology is most likely multifactorial involving contributions from environmental factors in addition to an important role for crucial developmental genes. This potentially complex interaction between genetics and the environment is borne out by the variability of the penetrance and phenotypes in patients with genetic SOD, but at present, the understanding of these interactions is rudimentary. Further study of these critical factors may shed light on the aetiology of this complex disorder.



Similar content being viewed by others
References
De Morsier G (1956) [Studies on malformation of cranio-encephalic sutures. III. Agenesis of the septum lucidum with malformation of the optic tract.]. Schweiz Arch Neurol Psychiatr 77(1–2):267–292
Reeves DL (1941) Congenital absence of the septum pellucidum. Bull Johns Hopkins Hosp 69:61–71
Hoyt WF, Kaplan SL, Grumbach MM, Glaser JS (1970) Septo-optic dysplasia and pituitary dwarfism. Lancet 1(7652):893–894
Arslanian SA, Rothfus WE, Foley TP Jr, Becker DJ (1984) Hormonal, metabolic, and neuroradiologic abnormalities associated with septo-optic dysplasia. Acta Endocrinol (Copenh) 107(2):282–288
Izenberg N, Rosenblum M, Parks JS (1984) The endocrine spectrum of septo-optic dysplasia. Clin Pediatr (Phila) 23(11):632–636
Roessmann U (1989) Septo-optic dysplasia (SOD) or DeMorsier syndrome. J Clin Neuroophthalmol 9(3):156–159
Stanhope R, Preece MA, Brook CG (1984) Hypoplastic optic nerves and pituitary dysfunction. A spectrum of anatomical and endocrine abnormalities. Arch Dis Child 59(2):111–114
Patel L, McNally RJ, Harrison E, Lloyd IC, Clayton PE (2006) Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr 148(1):85–88
Kuriyama M, Shigematsu Y, Konishi K, Konishi Y, Sudo M, Haruki S, Ito H (1988) Septo-optic dysplasia with infantile spasms. Pediatr Neurol 4(1):62–65
Miller SP, Shevell MI, Patenaude Y, Poulin C, O’Gorman AM (2000) Septo-optic dysplasia plus: a spectrum of malformations of cortical development. Neurology 54(8):1701–1703
Brodsky MC, Glasier CM (1993) Optic nerve hypoplasia. Clinical significance of associated central nervous system abnormalities on magnetic resonance imaging [published erratum appears in Arch Ophthalmol 1993 Apr;111(4):491]. Arch Ophthalmol 111(1):66–74
Zeki SM, Hollman AS, Dutton GN (1992) Neuroradiological features of patients with optic nerve hypoplasia. J Pediatr Ophthalmol Strabismus 29(2):107–112
Shammas NW, Brown JD, Foreman BW, Marutani DR, Maddela D, Tonner D (1993) Septo-optic dysplasia associated with polyendocrine dysfunction. J Med 24(1):67–74
Willnow S, Kiess W, Butenandt O, Dorr HG, Enders A, Strasser-Vogel B, Egger J, Schwarz HP (1996) Endocrine disorders in septo-optic dysplasia (De Morsier syndrome) – evaluation and follow up of 18 patients. Eur J Pediatr 155(3):179–184
Acers TE (1981) Optic nerve hypoplasia: septo-optic-pituitary dysplasia syndrome. Trans Am Ophthalmol Soc 79:425–457
Birkebaek NH, Patel L, Wright NB, Grigg JR, Sinha S, Hall CM, Price DA, Lloyd IC, Clayton PE (2003) Endocrine status in patients with optic nerve hypoplasia: relationship to midline central nervous system abnormalities and appearance of the hypothalamic-pituitary axis on magnetic resonance imaging. J Clin Endocrinol Metab 88(11):5281–5286
Cameron FJ, Khadilkar VV, Stanhope R (1999) Pituitary dysfunction, morbidity and mortality with congenital midline malformation of the cerebrum. Eur J Pediatr 158(2):97–102
Costin G, Murphree AL (1985) Hypothalamic-pituitary function in children with optic nerve hypoplasia. Am J Dis Child 139(3):249–254
Freude S, Frisch H, Wimberger D, Schober E, Husler G, Waldhauser F, Aichner F (1992) Septo-optic dysplasia and growth hormone deficiency: accelerated pubertal maturation during GH therapy. Acta Paediatr 81(8):641–645
Huseman CA, Kelch RP, Hopwood NJ, Zipf WB (1978) Sexual precocity in association with septo-optic dysplasia and hypothalamic hypopituitarism. J Pediatr 92(5):748–753
Hanna CE, Mandel SH, LaFranchi SH (1989) Puberty in the syndrome of septo-optic dysplasia. Am J Dis Child 143(2):186–189
Lam KS, Wang C, Ma JT, Leung SP, Yeung RT (1986) Hypothalamic defects in two adult patients with septo-optic dysplasia. Acta Endocrinol (Copenh) 112(3):305–309
Yukizane S, Kimura Y, Yamashita Y, Matsuishi T, Horikawa H, Ando H, Yamashita F (1990) Growth hormone deficiency of hypothalamic origin in septo-optic dysplasia. Eur J Pediatr 150(1):30–33
Roessmann U, Velasco ME, Small EJ, Hori A (1987) Neuropathology of “septo-optic dysplasia” (de Morsier syndrome) with immunohistochemical studies of the hypothalamus and pituitary gland. J Neuropathol Exp Neurol 46(5):597–608
Masera N, Grant DB, Stanhope R, Preece MA (1994) Diabetes insipidus with impaired osmotic regulation in septo-optic dysplasia and agenesis of the corpus callosum. Arch Dis Child 70(1):51–53
Morishima A, Aranoff GS (1986) Syndrome of septo-optic-pituitary dysplasia: the clinical spectrum. Brain Dev 8(3):233–239
McNay DE, Turton JP, Kelberman D, Woods KS, Brauner R, Papadimitriou A, Keller E, Keller A, Haufs N, Krude H, Shalet SM, Dattani MT (2006) HESX1 mutations are an uncommon cause of septo-optic dysplasia and hypopituitarism. J Clin Endocrinol Metab 92(2):691–697
Zaias B, Becker D (1978) Septo-optic dysplasia: developmental or acquired abnormality? A case report. Trans Am Neurol Assoc 103:273–277
Thomas P, Brickman JM, Popperl H, Krumlauf R, Beddington RS (1997) Axis duplication and anterior identity in the mouse embryo. Cold Spring Harb Symp Quant Biol 62:115–125
Wales JK, Quarrell OW (1996) Evidence for possible Mendelian inheritance of septo-optic dysplasia. Acta Paediatr 85(3):391–392
Blethen SL, Weldon VV (1985) Hypopituitarism and septooptic “dysplasia” in first cousins. Am J Med Genet 21(1):123–129
Benner JD, Preslan MW, Gratz E, Joslyn J, Schwartz M, Kelman S (1990) Septo-optic dysplasia in two siblings. Am J Ophthalmol 109(6):632–637
Thomas PQ, Dattani MT, Brickman JM, McNay D, Warne G, Zacharin M, Cameron F, Hurst J, Woods K, Dunger D, Stanhope R, Forrest S, Robinson IC, Beddington RS (2001) Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet 10(1):39–45
Cohen RN, Cohen LE, Botero D, Yu C, Sagar A, Jurkiewicz M, Radovick S (2003) Enhanced repression by HESX1 as a cause of hypopituitarism and septooptic dysplasia. J Clin Endocrinol Metab 88(10):4832–4839
Tajima T, Hattorri T, Nakajima T, Okuhara K, Sato K, Abe S, Nakae J, Fujieda K (2003) Sporadic heterozygous frameshift mutation of HESX1 causing pituitary and optic nerve hypoplasia and combined pituitary hormone deficiency in a Japanese patient. J Clin Endocrinol Metab 88(1):45–50
Beddington RS, Robertson EJ (1999) Axis development and early asymmetry in mammals. Cell 96(2):195–209
Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Martensson IL, Toresson H, Fox M, Wales JKH, Hindmarsh PC, Krauss S, Beddington RSP, Robinson ICAF (1998) Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet 19(2):125–133
Shawlot W, Behringer RR (1995) Requirement for Lim1 in head-organizer function. Nature 374(6521):425–430
Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J (1996) A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122(1):243–252
Rubenstein JL, Shimamura K, Martinez S, Puelles L (1998) Regionalization of the prosencephalic neural plate. Annu Rev Neurosci 21:445–477
Couly G, Le Douarin NM (1988) The fate map of the cephalic neural primordium at the presomitic to the 3-somite stage in the avian embryo. Development 103(Suppl):101–113
Eagleson GW, Harris WA (1990) Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol 21(3):427–440
Jacobson AG, Miyamoto DM, Mai SH (1979) Rathke’s pouch morphogenesis in the chick embryo. J Exp Zool 207:351–366
Dasen JS, Rosenfeld MG (1999) Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr Opin Cell Biol 11(6):669–677
Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ (1996) The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10(1):60–69
Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG (1998) Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12(11):1691–1704
Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan LM, Pfaff SL, Westphal H, Kimura S, Mahon KA (1998) Formation of Rathke’s pouch requires dual induction from the diencephalon. Development 125(23):4835–4840
Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P (1995) Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121(12):4045–4055
Walther C, Gruss P (1991) Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113(4):1435–1449
Thomas PQ, Johnson BV, Rathjen J, Rathjen PD (1995) Sequence, genomic organization, and expression of the novel homeobox gene Hesx1. J Biol Chem 270(8):3869–3875
Hermesz E, Mackem S, Mahon KA (1996) Rpx: a novel anterior-restricted homeobox gene progressively activated in the prechordal plate, anterior neural plate and Rathke’s pouch of the mouse embryo. Development 122(1):41–52
Zhadanov AB, Bertuzzi S, Taira M, Dawid IB, Westphal H (1995) Expression pattern of the murine LIM class homeobox gene Lhx3 in subsets of neural and neuroendocrine tissues. Dev Dyn 202(4):354–364
Bach I, Rhodes SJ, Pearse RV, Heinzel T, Gloss B, Scully KM, Sawchenko PE, Rosenfeld MG (1995) P-Lim, a LIM homeodomain factor, is expressed during pituitary organ and cell commitment and synergizes with Pit-1. Proc Natl Acad Sci USA 92(7):2720–2724
Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H (1997) Multistep control of pituitary organogenesis. Science 278(5344):1809–1812
Sheng HZ, Zhadanov AB, Mosinger B, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H (1996) Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272(5264):1004–1007
Lamonerie T, Tremblay JJ, Lanctot C, Therrien M, Gauthier Y, Drouin J (1996) Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev 10(10):1284–1295
Szeto DP, Ryan AK, O’Connell SM, Rosenfeld MG (1996) P-OTX: a PIT-1-interacting homeodomain factor expressed during anterior pituitary gland development. Proc Natl Acad Sci USA 93(15):7706–7710
Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW (1990) Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 4(5):695–711
Japon MA, Rubinstein M, Low MJ (1994) In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem 42(8):1117–1125
Li S, Crenshaw EB III, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG (1990) Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347(6293):528–533
Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384(6607):327–333
Wu W, Cogan JD, Pfaffle RW, Dasen JS, Frisch H, O’Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA III, Rosenfeld MG (1998) Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet 18(2):147–149
Artman HG, Boyden E (1990) Microphthalmia with single central incisor and hypopituitarism. J Med Genet 27(3):192–193
Brook CG, Sanders MD, Hoare RD (1972) Septo-optic dysplasia. Br Med J 3(830):811–813
Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA (1993) Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol 50(7):771–775
Fitz CR (1994) Holoprosencephaly and septo-optic dysplasia. Neuroimaging Clin N Am 4(2):263–281
Thomas P, Beddington R (1996) Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol 6(11):1487–1496
Dasen JS, Barbera JP, Herman TS, Connell SO, Olson L, Ju B, Tollkuhn J, Baek SH, Rose DW, Rosenfeld MG (2001) Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev 15(23):3193–3207
Dasen JS, Rosenfeld MG (2001) Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci 24:327–355
Brickman JM, Clements M, Tyrell R, McNay D, Woods K, Warner J, Stewart A, Beddington RS, Dattani M (2001) Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders. Development 128(24):5189–5199
Carvalho LR, Woods KS, Mendonca BB, Marcal N, Zamparini AL, Stifani S, Brickman JM, Arnhold IJ, Dattani MT (2003) A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction. J Clin Invest 112(8):1192–1201
Sobrier ML, Netchine I, Heinrichs C, Thibaud N, Vie-Luton MP, Van Vliet G, Amselem S (2005) Alu-element insertion in the homeodomain of HESX1 and aplasia of the anterior pituitary. Hum Mutat 25(5):503
Sobrier ML, Maghnie M, Vie-Luton MP, Secco A, di Iorgi N, Lorini R, Amselem S (2006) Novel HESX1 mutations associated with a life-threatening neonatal phenotype, pituitary aplasia, but normally located posterior pituitary and no optic nerve abnormalities. J Clin Endocrinol Metab 91(11):4528–4536
Tornqvist K, Ericsson A, Kallen B (2002) Optic nerve hypoplasia: risk factors and epidemiology. Acta Ophthalmol Scand 80(3):300–304
Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346(6281):245–250
Stevanovic M, Lovell-Badge R, Collignon J, Goodfellow PN (1993) SOX3 is an X-linked gene related to SRY. Hum Mol Genet 2(12):2013–2018
Pevny LH, Lovell-Badge R (1997) Sox genes find their feet. Curr Opin Genet Dev 7(3):338–344
Wegner M (1999) From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27(6):1409–1420
Bowles J, Schepers G, Koopman P (2000) Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol 227(2):239–255
Stevanovic M, Zuffardi O, Collignon J, Lovell-Badge R, Goodfellow P (1994) The cDNA sequence and chromosomal location of the human SOX2 gene. Mamm Genome 5(10):640–642
Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H (1998) Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125(13):2521–2532
Denny P, Swift S, Brand N, Dabhade N, Barton P, Ashworth A (1992) A conserved family of genes related to the testis determining gene, SRY. Nucleic Acids Res 20(11):2887
Mertin S, McDowall SG, Harley VR (1999) The DNA-binding specificity of SOX9 and other SOX proteins. Nucleic Acids Res 27(5):1359–1364
Foster JW, Graves JA (1994) An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene. Proc Natl Acad Sci USA 91(5):1927–1931
Wood HB, Episkopou V (1999) Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev 86(1–2):197–201
Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122(2):509–520
Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R (1998) A role for SOX1 in neural determination. Development 125(10):1967–1978
Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK (2000) Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development 127(11):2367–2382
Pevny L, Placzek M (2005) SOX genes and neural progenitor identity. Curr Opin Neurobiol 15(1):7–13
Bylund M, Andersson E, Novitch BG, Muhr J (2003) Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci 6(11):1162–1168
Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R (2004) SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet 36(3):247–255
Weiss J, Meeks JJ, Hurley L, Raverot G, Frassetto A, Jameson JL (2003) Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol 23(22):8084–8091
Rizzoti K, Lovell-Badge R (2005) Early development of the pituitary gland: induction and shaping of Rathke’s pouch. Rev Endocr Metab Disord 6(3):161–172
Ericson J, Norlin S, Jessell TM, Edlund T (1998) Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125(6):1005–1015
Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE (1999) Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell 4(4):487–498
Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH Jr, MacDougald OA, Camper SA (2001) Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome 12(11):843–851
Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA (2004) WNT5A signaling affects pituitary gland shape. Mech Dev 121(2):183–194
Hamel BC, Smits AP, Otten BJ, van den Helm HB, Ropers HH, Mariman EC (1996) Familial X-linked mental retardation and isolated growth hormone deficiency: clinical and molecular findings. Am J Med Genet 64(1):35–41
Lagerstrom-Fermer M, Sundvall M, Johnsen E, Warne GL, Forrest SM, Zajac JD, Rickards A, Ravine D, Landegren U, Pettersson U (1997) X-linked recessive panhypopituitarism associated with a regional duplication in Xq25-q26. Am J Hum Genet 60(4):910–916
Hol FA, Schepens MT, van Beersum SE, Redolfi E, Affer M, Vezzoni P, Hamel BC, Karnes PS, Mariman EC, Zucchi I (2000) Identification and characterization of an Xq26-q27 duplication in a family with spina bifida and panhypopituitarism suggests the involvement of two distinct genes. Genomics 69(2):174–181
Solomon NM, Nouri S, Warne GL, Lagerstrom-Fermer M, Forrest SM, Thomas PQ (2002) Increased gene dosage at Xq26-q27 is associated with X-linked hypopituitarism. Genomics 79(4):553–559
Stankiewicz P, Thiele H, Schlicker M, Cseke-Friedrich A, Bartel-Friedrich S, Yatsenko SA, Lupski JR, Hansmann I (2005) Duplication of Xq26.2-q27.1, including SOX3, in a mother and daughter with short stature and dyslalia. Am J Med Genet Part A 138A(1):11–17
Woods KS, Cundall M, Turton J, Rizotti K, Mehta A, Palmer R, Wong J, Chong WK, Al Zyoud M, El Ali M, Otonkoski T, Martinez-Barbera JP, Thomas PQ, Robinson IC, Lovell-Badge R, Woodward KJ, Dattani MT (2005) Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet 76(5):833–849
Laumonnier F, Ronce N, Hamel BCJ, Thomas P, Lespinasse J, Raynaud M, Paringaux C, Van Bokhoven H, Kalscheuer V, Fryns JP, Chelly J, Moraine C, Briault S (2002) Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet 71(6):1450–1455
Lim HN, Berkovitz GD, Hughes IA, Hawkins JR (2000) Mutation analysis of subjects with 46, XX sex reversal and 46, XY gonadal dysgenesis does not support the involvement of SOX3 in testis determination. Hum Genet 107(6):650–652
Raverot G, Lejeune H, Kotlar T, Pugeat M, Jameson JL (2004) X-linked sex-determining region Y box 3 (SOX3) gene mutations are uncommon in men with idiopathic oligoazoospermic infertility. J Clin Endocrinol Metab 89(8):4146–4148
Albrecht AN, Kornak U, Boddrich A, Suring K, Robinson PN, Stiege AC, Lurz R, Stricker S, Wanker EE, Mundlos S (2004) A molecular pathogenesis for transcription factor associated poly-alanine tract expansions. Hum Mol Genet 13(20):2351–2359
Bowl MR, Nesbit MA, Harding B, Levy E, Jefferson A, Volpi E, Rizzoti K, Lovell-Badge R, Schlessinger D, Whyte MP, Thakker RV (2005) An interstitial deletion-insertion involving chromosomes 2p25.3 and Xq27.1, near SOX3, causes X-linked recessive hypoparathyroidism. J Clin Invest 115(10):2822–2831
Williamson KA, Hever AM, Rainger J, Rogers RC, Magee A, Fiedler Z, Keng WT, Sharkey FH, McGill N, Hill CJ, Schneider A, Messina M, Turnpenny PD, Fantes JA, van Heyningen V, FitzPatrick DR (2006) Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum Mol Genet 15(9):1413–1422
Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17(1):126–140
Ferri ALM, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK (2004) Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131(15):3805–3819
Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH (2006) SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev 20(9):1187–1202
Kiernan AE, Pelling AL, Leung KKH, Tang ASP, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KSE (2005) Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434(7036):1031–1035
Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, Carmignac D, Lovell-Badge R, Robinson IC, Dattani MT (2006) Mutations withinSox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest 116:2442–2455
Fantes J, Ragge NK, Lynch SA, Mcgill NI, Collin JRO, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, FitzPatrick DR (2003) Mutations in SOX2 cause anophthalmia. Nat Genet 33(4):461–463
Ragge NK, Lorenz B, Schneider A, Bushby K, de Sanctis L, de Sanctis U, Salt A, Collin JRO, Vivian AJ, Free SL, Thompson P, Williamson KA, Sisodiya SM, van Heyningen V, FitzPatrick DR (2005) SOX2 anophthalmia syndrome. Am J Med Genet Part A 135A(1):1–7
Hagstrom SA, Pauer GJT, Reid J, Simpson E, Crowe S, Maumenee IH, Traboulsi EI (2005) SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am J Med Genet Part A 138A(2):95–98
Zenteno JC, Gascon-Guzman G, Tovilla-Canales JL (2005) Bilateral anophthalmia and brain malformations caused by a 20-bp deletion in the SOX2 gene. Clin Genet 68(6):564–566
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelberman, D., Dattani, M.T. Genetics of septo-optic dysplasia. Pituitary 10, 393–407 (2007). https://doi.org/10.1007/s11102-007-0055-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-007-0055-5