Abstract
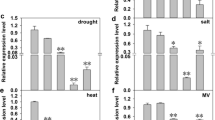
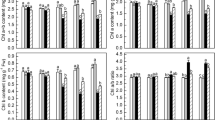
We studied how tomato (Lycopersicon esculentum Mill.) chloroplast omega-3 fatty acid desaturase gene (Lefad7) overexpression enhanced low-temperature (LT) tolerance in transgenic tomato plants. In these plants, the content of linolenic acid (18:3) markedly increased and, correspondingly, the content of linoleic acid (18:2) decreased. Similar changes were found after 6 h under LT (4°C) treatment. Under LT stress, wild type (WT) tomato plants showed a much greater increase in relative electrolyte leakage and malondialdehyde (MDA) contents compared with transgenic plants. Transgenic plants exhibited higher activities of antioxidative enzymes and a lower content of reactive oxygen species (ROS). Transgenic plants maintained a relatively higher level of the net photosynthetic rate (P N) and chlorophyll (Chl) content than WT plants under LT stress. Taken together, we suggested that overexpression of Lefad7 enhanced LT tolerance by changing the composition of membrane lipids in tomato plants, with the increased content of trienoic fatty acids and reduced content of dienoic fatty acids that led to series of physiological alterations.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- AtFAD7 :
-
Arabidopsis thaliana omega-3 fatty acid desaturase
- Chl:
-
chlorophyll
- DAs:
-
dienoic fatty acids
- DGDG:
-
digalactosyldiacylglycerol
- FADs:
-
fatty acid desaturases
- IUFA:
-
index of unsaturated fatty acid
- Lefad7 :
-
Lycopersicon esculentum omega-3 fatty acid desaturase gene
- LT:
-
low-temperature
- MDA:
-
malondialdehyde
- MGDG:
-
monogalactosyldiacylglycerol
- PFD:
-
photon flux density
- PG:
-
phosphatidylglycerol
- P N :
-
net photosynthetic rate
- PUFAs:
-
polyunsaturated fatty acids
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- SQDG:
-
ulfoquinovosyldiacylglycerol
- TAs:
-
trienoic fatty acids
- WT:
-
wild type
- 16:1(3t):
-
trans-hexadecenoic acids
- 16:3:
-
hexadecatrienoic acids
- 18:1:
-
oleic acid
- 18:2:
-
linoleic acid
- 18:3:
-
linolenic acids
References
Andreu, V., Collados, R., Testillano, P.S. et al.: In situ molecular identification of the plastid omega3 fatty acid desaturase FAD7 from soybean: evidence of thylakoid membrane localization. — Plant Physiol. 145: 1336–1344, 2007.
Andreu, V., Lagunas, B., Collados, R., et al.: The GmFAD7 gene family from soybean: identification of novel genes and tissue-specific conformations of the FAD7 enzyme involved in desaturase activity. — J. Exp. Bot. 61: 3371–3384, 2010.
Alonso, A., Queiroz, C.S., Magalhaes, A.C.: Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings. — Biochim. Biophys. Acta 1323: 75–84, 1997.
Barclay, K.D., McKersie, B.D.: Peroxidation reactions in plant membranes: effects of free fatty acids. — Lipids 29: 877–883, 1994.
Browse, J., Kunst, L., Anderson, S. et al.: A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. — Plant Physiol. 90: 522–529, 1989.
Browse, J., McCourt, P., Somerville, C.: A mutant of Arabidopsis deficient in c(18:3) and c(16:3) leaf lipids. — Plant Physiol. 81: 859–864, 1986.
Browse, J., Somerville, C.: Glycerolipid synthesis: biochemistry and regulation. — Annu. Rev. Plant Physiol. Plant Mol. Biol. 42: 467–506, 1991.
Bruinsma, J.: A Comment on spectrophotometric determination of chlorophyll. — Biochim. Biophys. Acta 52: 576–578, 1961.
Burdon, R.H., Gill, V., Boyd, P.A., O’Kane, D.: Chilling, Oxidative Stress and Antioxidant Enzyme Responses in Arabidopsis thaliana. — Proc. Roy. Soc. Edinburgh B-Biol. Sci. 102: 177–185, 1994.
Chapin, F.S., Zavaleta, E.S., Eviner, V.T. et al.: Consequences of changing biodiversity. — Nature 405: 234–242, 2000.
Cheesbrough, T.M.: Changes in the Enzymes for Fatty Acid Synthesis and Desaturation during Acclimation of Developing Soybean Seeds to Altered Growth Temperature. — Plant Physiol. 90: 760–764, 1989.
Fryer, M.J., Andrews, J.R., Oxborough, K. et al.: Relationship between CO2 Assimilation, Photosynthetic Electron Transport, and Active O2 Metabolism in Leaves of Maize in the Field during Periods of Low Temperature. — Plant Physiol. 116: 571–580, 1998.
Giannopolitis, C.N., Ries, S.K.: Superoxide Dismutases 1 Occurrence in Higher Plants. — Plant Physiol. 59: 309–314, 1977.
Griffiths, G., Leverentz, M., Silkowski, H., Gill, N., Sanchez-Serrano, J.J.: Lipid hydroperoxide levels in plant tissues. — J. Exp. Bot. 51: 1363–1370, 2000.
Guo, B., Liang, Y.C., Zhu, Y.G., Zhao, F.J.: Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. — Environ. Pollution 147: 743–749, 2007.
Hara, M., Terashima, S., Fukaya, T., Kuboi, T.: Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. — Planta 217: 290–298, 2003.
Huang, G., Ma, S., Bai, L. et al.: Signal transduction during cold, salt, and drought stresses in plants. — Mol. Biol. Rep. 39: 969–987, 2012.
Iba, K.: Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. — Annu. Rev. Plant Biol. 53: 225–245, 2002.
Jimenez, A., Hernandez, J.A., delRio, L.A., Sevilla, F.: Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. — Plant Physiol. 114: 275–284, 1997.
Kargiotidou, A., Deli, D., Galanopoulou, D., Tsaftaris, A., Farmaki, T.: Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum). — J. Exp. Bot. 59: 2043–2056, 2008.
Kodama, H., Hamada, T., Horiguchi, G. et al.: Genetic Enhancement of Cold Tolerance by Expression of a Gene for Chloroplast [omega]-3 Fatty Acid Desaturase in Transgenic Tobacco. — Plant Physiol. 105: 601–605, 1994.
Kratsch, H.A., Wise, R.R.: The ultrastructure of chilling stress. — Plant Cell Environ. 23: 337–350, 2000.
Lemieux, B., Miquel, M., Somerville, C., Browse, J.: Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. — Theor. Appl. Genet. 80: 232–240, 1990.
Liu, X.Y., Li, B., Yang, J.H. et al.: Overexpression of tomato chloroplast omega-3 fatty acid desaturase gene alleviates the photoinhibition of photosystems 2 and 1 under chilling stress. — Photosynthetica 46: 185–192, 2008.
Liu, X.Y., Yang, J.H., Li, B. et al.: Antisense-mediated depletion of tomato chloroplast omega-3 fatty acid desaturase enhances thermal tolerance. — J. Integrative Plant Biol. 48: 1096–1107, 2006.
McConn, M., Hugly, S., Browse, J., Somerville, C.: A Mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. — Plant Physiol. 106: 1609–1614, 1994.
Miquel, M., Browse, J.: Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. — J. Biol. Chem. 267: 1502–1509, 1992.
Mittler, R.: Oxidative stress, antioxidants and stress tolerance. — Trends Plant Sci. 7: 405–410, 2002.
Murakami, Y., Tsuyama, M., Kobayashi, Y. et al.: Trienoic fatty acids and plant tolerance of high temperature. — Science 287: 476–479, 2000.
Murata, N., Los, D.A.: Membrane Fluidity and Temperature Perception. — Plant Physiol. 115: 875–879, 1997.
Nishida, I., Murata, N.: Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. — Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 541–568, 1996.
Nishiuchi, T., Iba, K.: Roles of plastid omega-3 fatty acid desaturases in defense response of higher plants. — J. Plant Res. 111: 481–486, 1998.
O’Kane, D., Gill, V., Boyd, P., Burdon, B.: Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. — Planta 198: 371–377, 1996.
Routaboul, J.M., Fischer, S.F., Browse, J.: Trienoic fatty acids are required to maintain chloroplast function at low temperatures. — Plant Physiol. 124: 1697–1705, 2000.
Sairam, R.K., Srivastava, G.C.: Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. — Plant Sci. 162: 897–904, 2002.
Santos, C.L.V., Campos, A., Azevedo, H., Caldeira, G.: In situ and in vitro senescence induced by KCl stress: nutritional imbalance, lipid peroxidation and antioxidant metabolism. — J. Exp. Bot. 52: 351–360, 2001.
Shimada, T., Wakita, Y., Otani, M., Iba, K.: Modification of fatty acid composition in rice plants by transformation with a tobacco microsomal omega-3 fatty acid desaturase gene (NtFAD3). — Plant Biotech. 17: 43–48, 2000.
Somerville, C.: Direct tests of the role of membrane lipid composition in low-temperature-induced photoinhibition and chilling sensitivity in plants and cyanobacteria. — Proc. Natl. Acad. Sci. USA 92: 6215–6218, 1995.
Somerville, C., Browse, J.: Dissecting desaturation: plants prove advantageous. — Trends Cell Biol. 6: 148–153, 1996.
Su, W.A., Wang, W.Y., Li, J.S.: Analysis of plant lipid and fatty acid. — Plant Physiol. Commun. 3: 54–60, 1980.
Teixeira, M.C., Carvalho, I.S., Brodelius, M.: Omega-3 fatty acid desaturase genes isolated from purslane (Portulaca oleracea L.): expression in different tissues and response to cold and wound stress. — J. Agric. Food Chem. 58: 1870–1877, 2010.
Upchurch, R.G.: Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. — Biotechnol. Lett. 30: 967–977, 2008.
Wang, A.G., Luo, G.H.: Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. — Plant Physiol. Commun. 6: 55–57, 1990.
Williams, J.P., Khan, M.U., Wong, D.: Low temperatureinduced fatty acid desaturation in Brassica napus: thermal deactivation and reactivation of the process. — Biochim. Biophys. Acta. 1128: 275–279, 1992.
Xu, Y.N., Siegenthaler, P.A.: Low temperature treatments induce an increase in the relative content of both linolenic and delta(3)-trans-hexadecenoic acid in thylakoid membrane phosphatidylglycerol of squash cotyledons. — Plant Cell Physiol. 38: 611–618, 1997.
Yu, C., Wang, H.S., Yang, S. et al.: Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. — Plant Physiol. Biochem. 47: 1102–1112, 2009.
Zhang, M., Barg, R., Yin, M. et al.: Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. — Plant J. 44: 361–371, 2005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This research was supported by a grant from the Major State Basic Research Development Program of China (973 program) (No. 2009CB118505) and the Natural Science Foundation of Zhejiang Province (Y3110327).
Rights and permissions
About this article
Cite this article
Liu, X.Y., Teng, Y.B., Li, B. et al. Enhancement of low-temperature tolerance in transgenic tomato plants overexpressing Lefad7 through regulation of trienoic fatty acids. Photosynthetica 51, 238–244 (2013). https://doi.org/10.1007/s11099-013-0014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-013-0014-5