Abstract
Background
It is a common cost-containment practice in some countries to dispense a cheaper, generic version of a prescribed medication. This presents few problems for most medications. However, dry powder inhalers used in asthma and COPD vary markedly in design and method of operation, so generic substitution may not be acceptable to patients or healthcare professionals. Patients dispensed an unfamiliar device in which they have received no training, risk poor inhalation technique with the potential for inadequate dosing and loss of disease control.
Objective
To assess the views of pharmacists towards interchangeable use of dry powder inhalers.
Setting
Community pharmacists in Australia, Canada, France, Germany, and the UK.
Method
Following exploration of the key issues with international opinion leaders in respiratory management, a structured web questionnaire was developed for use in computer assisted web interviews. Fieldwork was carried out in March and April 2005. Main outcome measure: Responses to the web questionnaire were analysed by percentage of respondents or by mean or median score, as appropriate to the question.
Results
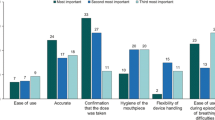
A total of 254 pharmacists were included in the study. Just 6% of pharmacists considered that dry powder inhalers are interchangeable, with a high level of concern shown about interchangeable use (median score of 6 on a scale of 1, not at all concerned, to 7, extremely concerned). Patient confusion was the main concern, expressed by 77% of respondents. Pharmacists also envisaged substitution having an adverse impact on pharmacy stock levels (72%), patient device handling (70%), pharmacist workload (63%), patient compliance (56%) and outcomes for the patient (51%), with pharmacists in Germany having a particularly negative view and those in France generally the most positive. Despite the generally negative view of pharmacists about interchangeable use of dry powder inhalers, overall only 22% would contact the prescribing physician often/very often for approval of the substitution.
Conclusion
The study showed that only a small minority of pharmacists believe that dry powder inhalers can be used interchangeably, with the majority concerned about generic substitution of these products. Pharmacists in Germany were particularly negative about the interchangeable use of dry powder inhalers.
Similar content being viewed by others
References
Chrystyn H. Do patients show the same level of adherence with all dry powder inhalers? Int J Clin Pract 2005;59(Suppl 149):19–25
Labris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. J Clin Pharmacol 2003;56:600–12
Gustafsson P, Taylor A, Zanen P, Chrystyn H. Can patients use all dry powder inhalers equally well? Int J Clin Pract 2005;59(Suppl 149):13–8
Buchanan A, Pinnock H, Barnes J et al. Generic prescribing of breath actuated and dry powder inhalers in the UK. Prim Care Respir J 2002;11:95
Bisgaard H. Future options for aerosol delivery to children. Allergy 1999;54:97–103
Taylor A, Gustafsson P. Do all dry powder inhalers show the same pharmaceutical performance?. Int J Clin Pract 2005;59(Suppl 149):7–12
Pritchard JN. The influence of lung deposition on clinical response. J Aerosol Med 2001;14(S1):S19–S26
Bateman ED, Silins V, Bogolubov M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 mcg twice daily) when administered via a chlorofluorocarbon-free metered dose inhaler or dry powder inhaler to patients with mild-to-moderate asthma. Respir Med 2001;95:136–46
BTS/SIGN (British Thoracic Society/Scottish Intercollegiate Guidelines Network). British guideline on the management of asthma. Thorax 2003;58(suppl 1):i1–i94
GINA (Global Initiative for asthma). Pocket guide for asthma management and prevention. http://www.ginasthma.com/Guidelineitem.asp?l1=2&l2=1&intId=988
Thomas M, Williams AE. Are outcomes the same with all dry powder inhalers? Int J Clin Pract 2005;59(Suppl 149):33–5
Price D. Do healthcare professionals think that dry powder inhalers can be used interchangeably? Int J Clin Pract 2005;59(Suppl 149): 26–9
Booker R. Do patients think that dry powder inhalers can be used interchangeably? Int J Clin Pract 2005;59(Suppl 149): 30–2
Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J 1996;9:636–42
Hoskins G, McCowan C, Neville RG et al. Risk factors and costs associated with an asthma attack. Thorax 2000;55:19–24
Adams WP, Puchikian G, Taylor AS, Patel RM, Burke GP, Williams RL. Regulatory aspects of modification to innovator bronchodilator metered dose inhalers and development of generic substitutes. J Aerosol Med 1994;7:119–34
Price D, Summers M, Zanen P. Could interchangeable use of dry powder inhalers affect patients?. Int J Clin Pract 2005;59(Suppl 149):3–6
Wood KM, Boath EH, Mucklow JC, Blenkinsopp A. Changing medication: general practitioner and patient perspectives. Int J Phar Pract 1997;5:176–84
Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freemantle N, Vail A. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomized controlled trial. Health Technol Assess 2002;6:1–86
Vidgren M, Kärkkäinen A, Karjalainen P et al. Effect of powder inhaler design on drug deposition in the respiratory tract. Int J Pharm 1988;42:211–6
Thorsson L, Edsbacker S. Less variability in lung deposition of budesonide via Turbuhaler than of fluticasone via Diskus/Accuhaler and pMDI in adults. Am J Respir Crit Care Med 2003;167(Suppl 7):A896
Kesten S, Zive K, Chapman KR. Pharmacist knowledge and ability to use inhaled medication delivery systems. Chest 1993;104:1737–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, A.E., Chrystyn, H. Survey of pharmacists’ attitudes towards interchangeable use of dry powder inhalers. Pharm World Sci 29, 221–227 (2007). https://doi.org/10.1007/s11096-006-9079-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-006-9079-6