Abstract
Purpose
The purpose of this work is to evaluate the interrelationship of microstructure, properties, and dissolution performance for amorphous solid dispersions (ASDs) prepared using different methods.
Methods
ASD of GDC-0810 (50% w/w) with HPMC-AS was prepared using methods of spray drying and co-precipitation via resonant acoustic mixing. Microstructure, particulate and bulk powder properties, and dissolution performance were characterized for GDC-0810 ASDs. In addition to application of typical physical characterization tools, we have applied X-Ray Microscopy (XRM) to assess the contribution of microstructure to the characteristics of ASDs and obtain additional quantification and understanding of the drug product intermediates and tablets.
Results
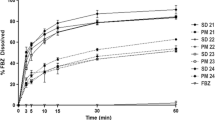
Both methods of spray drying and co-precipitation produced single-phase ASDs. Distinct differences in microstructure, particle size distribution, specific surface area, bulk and tapped density, were observed between GDC-0810 spray dried dispersion (SDD) and co-precipitated amorphous dispersion (cPAD) materials. The cPAD powders prepared by the resonant acoustic mixing process demonstrated superior compactibility compared to the SDD, while the compressibility of the ASDs were comparable. Both SDD powder and tablets showed higher in vitro dissolution than those of cPAD powders. XRM calculated total solid external surface area (SA) normalized by calculated total solid volume (SV) shows a strong correlation with micro dissolution data.
Conclusion
Strong interrelationship of microstructure, physical properties, and dissolution performance was observed for GDC-0810 ASDs. XRM image-based analysis is a powerful tool to assess the contribution of microstructure to the characteristics of ASDs and provide mechanistic understanding of the interrelationship.









Similar content being viewed by others
Abbreviations
- ASD:
-
Amorphous solid dispersion
- cPAD:
-
Co-precipitated amorphous dispersion
- FaSSIF-V2:
-
Fasted-state simulated intestinal fluid version 2
- HME:
-
Hot-melt extrusion
- SD:
-
Spray drying
- SDD:
-
Spray dried dispersion
- RAM:
-
Resonant acoustic mixing
- VDD:
-
Vacuum drum drying
- XRM:
-
X-ray microscopy
References
Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Curr Pharm Des. 2009;15(19):2184–94.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–47.
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17(9–10):486–95.
Vaka SR, Bommana MM, Desai D, Djordjevic J, Phuapradit W, Shah N. Excipients for amorphous solid dispersions. In amorphous solid dispersions 2014. p. 123-161. Springer, New York, NY.
Miller JM, Beig A, Carr RA, Spence JK, Dahan A. A win–win solution in oral delivery of lipophilic drugs: supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol Pharm. 2012;9(7):2009–16.
Shah N, Iyer RM, Mair HJ, Choi D, Tian H, Diodone R, Fahnrich K, Pabst-Ravot A, Tang K, Scheubel E, Grippo JF. Improved human bioavailability of vemurafenib, a practically insoluble drug, using an amorphous polymer-stabilized solid dispersion prepared by a solvent-controlled coprecipitation process. J Pharm Sci. 2013;102(3):967–81.
Bhujbal SV, Mitra B, Jain U, Gong Y, Agrawal A, Karki S, Taylor LS, Kumar S, Zhou QT. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm Sin B. 2021;11(8):2505–36.
Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, Mohammed NN. Melt extrusion: process to product. Expert Opin Drug Discov. 2012;9(1):105–25.
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42.
DiNunzio JC, Brough C, Miller DA, Williams RO III, McGinity JW. Applications of KinetiSol® dispersing for the production of plasticizer free amorphous solid dispersions. Eur J Pharm Sci. 2010;40(3):179–87.
DiNunzio JC, Brough C, Miller DA, Williams RO III, McGinity JW. Fusion processing of itraconazole solid dispersions by KinetiSol® dispersing: a comparative study to hot melt extrusion. J Pharm Sci. 2010;99(3):1239–53.
Ellenberger DJ, Miller DA, Williams RO. Expanding the application and formulation space of amorphous solid dispersions with KinetiSol®: a review. AAPS PharmSciTech. 2018;19(5):1933–56.
Alhijjaj M, Belton P, Qi S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur J Pharm Biopharm. 2016;108:111–25.
Tan DK, Maniruzzaman M, Nokhodchi A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modeling (FDM) 3D printing for personalised drug delivery. Pharmaceutics. 2018;10(4):203.
Moneghini M, Bellich B, Baxa P, Princivalle F. Microwave generated solid dispersions containing ibuprofen. Int J Pharm. 2008;361(1–2):125–30.
Doreth M, Hussein MA, Priemel PA, Grohganz H, Holm R, de Diego HL, Rades T, Löbmann K. Amorphization within the tablet: using microwave irradiation to form a glass solution in situ. Int J Pharm. 2017;519(1–2):343–51.
Fini A, Fernández-Hervás MJ, Holgado MA, Rodriguez L, Cavallari C, Passerini N, Caputo O. Fractal analysis of β-cyclodextrin–indomethacin particles compacted by ultrasound. J Pharm Sci. 1997;86(11):1303–9.
Guo Z, Boyce C, Rhodes T, Liu L, Salituro GM, Lee KJ, Bak A, Leung DH. A novel method for preparing stabilized amorphous solid dispersion drug formulations using acoustic fusion. Int J Pharm. 2021;592:120026.
International Council for Harmonisation FDA 2021. Q3C(R8) Impurities: Guidance for Residual Solvents.
Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50.
Nguyen DN, Clasen C, Van den Mooter G. Pharmaceutical applications of electrospraying. J Pharm Sci. 2016;105(9):2601–20.
Kwon HJ, Heo EJ, Kim YH, Kim S, Hwang YH, Byun JM, Cheon SH, Park SY, Kim DY, Cho KH, Maeng HJ. Development and evaluation of poorly water-soluble celecoxib as solid dispersions containing nonionic surfactants using fluidized-bed granulation. Pharmaceutics. 2019;11(3):136.
Pasquali I, Bettini R, Giordano F. Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals. Adv Drug Deliv Rev. 2008;60(3):399–410.
Wang ZL, Finlay WH, Peppler MS, Sweeney LG. Powder formation by atmospheric spray-freeze-drying. Powder Technol. 2006;170(1):45–52.
Schönfeld B, Westedt U, Wagner KG. Vacuum drum drying–a novel solvent-evaporation based technology to manufacture amorphous solid dispersions in comparison to spray drying and hot melt extrusion. Int J Pharm. 2021;596:120233.
Huang S, Williams RO. Effects of the preparation process on the properties of amorphous solid dispersions. AAPS PharmSciTech. 2018;19(5):1971–84.
Song S, Wang C, Wang S, Siegel RA, Sun CC. Efficient development of sorafenib tablets with improved oral bioavailability enabled by coprecipitated amorphous solid dispersion. Int J Pharm. 2021;610:121216.
Mann AK, Schenck L, Koynov A, Rumondor AC, Jin X, Marota M, Dalton C. Producing amorphous solid dispersions via co-precipitation and spray drying: impact to physicochemical and biopharmaceutical properties. J Pharm Sci. 2018;107(1):183–91.
Vasconcelos T, Marques S, das Neves J, Sarmento B. Amorphous solid dispersions: rational selection of a manufacturing process. Adv Drug Deliv Rev. 2016;100:85–101.
Davis MT, Potter CB, Walker GM. Downstream processing of a ternary amorphous solid dispersion: the impacts of spray drying and hot melt extrusion on powder flow, compression and dissolution. Int J Pharm. 2018;544(1):242–53.
Schönfeld BV, Westedt U, Wagner KG. Compression of amorphous solid dispersions prepared by hot-melt extrusion, spray drying and vacuum drum drying. Int J Pharm: X. 2021;3:100102.
Hou HH, Rajesh A, Pandya KM, Lubach JW, Muliadi A, Yost E, Jia W, Nagapudi K. Impact of method of preparation of amorphous solid dispersions on mechanical properties: comparison of coprecipitation and spray drying. J Pharm Sci. 2019;108(2):870–9.
Schomberg AK, Diener A, Wunsch I, Finke JH, Kwade A. The use of X-ray microtomography to investigate the microstructure of pharmaceutical tablets: potentials and comparison to common physical methods. Int J Pharm. 2021;3:100090.
Yost E, Chalus P, Zhang S, Peter S, Narang AS. Quantitative X-ray microcomputed tomography assessment of internal tablet defects. J Pharm Sci. 2019;108(5):1818–30. https://doi.org/10.1016/j.xphs.2018.12.024.
Zhu et al. Investigation of quantitative X-ray microscopy for assessment of API and excipient microstructure evolution in solid dosage processing. AAPS PharmSciTech. In review, 2022.
Xi H, Zhu A, Klinzing GR, Zhou L, Zhang S, Gmitter AJ, et al. Characterization of spray dried particles through microstructural imaging. J Pharm Sci. 2020;109(11):3404–12. https://doi.org/10.1016/j.xphs.2020.07.032.
Gamble JF, Tobyn M, Zhang S, Zhu A, Salplachta J, Matula J, et al. Characterization of the morphological nature of hollow spray dried dispersion particles using X-ray submicron-computed tomography. AAPS PharmSciTech. 2021;23(1):40. https://doi.org/10.1208/s12249-021-02184-7.
Zhang S, Stroud PA, Zhu A, Johnson MJ, Lomeo J, Burcham CL, et al. Characterizing the impact of spray dried particle morphology on tablet dissolution using quantitative X-ray microscopy. Eur J Pharm Sci. 2021;165:105921. https://doi.org/10.1016/j.ejps.2021.105921.
Hou HH, Jia W, Liu L, Cheeti S, Li J, Nauka E, Nagapudi K. Effect of microenvironmental pH modulation on the dissolution rate and oral absorption of the salt of a weak acid–case study of GDC-0810. Pharm Res. 2018;35(2):1–11.
Heckel RW. Density–pressure relationships in powder compaction. Trans Metall Soc AIME. 1961;221(4):671–5.
Zhang S, Byrnes AP, Jankovic J, Neilly J. Management, analysis, and simulation of micrographs with cloud computing. Microscopy Today. 2019;27(2):26–33.
Lehmann G, Legland D. Efficient N-dimensional surface estimation using Crofton formula and run-length encoding. The Insight Journal. 2012.
Zhang S, Byrne G. Characterization of transport mechanisms for controlled release polymer membranes using focused ion beam scanning electron microscopy image-based modeling. J Drug Deliv Sci Technol. 2021;61:102136.
Jankovic J, Zhang S, Putz A, Saha MS, Susac D. Multiscale imaging and transport modeling for fuel cell electrodes. J Mater Res. 2019;34(4):579–91.
Byrnes AP, Zhang S, Canter L, Sonnenfeld MD. Application of integrated core and 3D image rock physics to characterize Niobrara chalk properties including relative permeability with bound water effect. In Unconventional Resources Technology Conference, 2017, Austin, Texas.
Calahan JL, Azali SC, Munson EJ, Nagapudi K. Investigation of phase mixing in amorphous solid dispersions of AMG 517 in HPMC-AS using DSC, solid-state NMR, and solution calorimetry. Mol Pharm. 2015;12(11):4115–23.
Leuenberger H. The compressibility and compactibility of powder systems. Int J Pharm. 1982;12(1):41–55.
Vreeman G, Sun CC. Mean yield pressure from the in-die Heckel analysis is a reliable plasticity parameter. Int J Pharm. 2021;3:100094.
Yost E, Mazel V, Sluga K, Nagapudi K, Muliadi AR. Beyond Brittle/Ductile Classification: Applying Proper Constitutive Mechanical Metrics to Understand the Compression Characteristics of Pharmaceutical Materials. J Pharm Sci. 2022. In Press. https://doi.org/10.1016/j.xphs.2022.01.004.
Tye CK, Sun CC, Amidon GE. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J Pharm Sci. 2005;94(3):465–72.
Nandiyanto AB, Okuyama K. Progress in developing spray-drying methods for the production of controlled morphology particles: from the nanometer to submicrometer size ranges. Advanced Powder Technol. 2011;22(1):1–9.
Schenck L, Boyce C, Frank D, Koranne S, Ferguson HM, Strotman N. Hierarchical particle approach for co-precipitated amorphous solid dispersions for use in preclinical in vivo studies. Pharmaceutics. 2021;13(7):1034.
Esposito E, Roncarati R, Cortesi R, Cervellati F, Nastruzzi C. Production of Eudragit microparticles by spray-drying technique: influence of experimental parameters on morphological and dimensional characteristics. Pharm Dev Technol. 2000;5(2):267–78.
Shah N, Sandhu H, Phuapradit W, Pinal R, Iyer R, Albano A, Chatterji A, Anand S, Choi DS, Tang K, Tian H. Development of novel microprecipitated bulk powder (MBP) technology for manufacturing stable amorphous formulations of poorly soluble drugs. Int J Pharm. 2012;438(1–2):53–60.
Shah UV, Karde V, Ghoroi C, Heng JY. Influence of particle properties on powder bulk behaviour and processability. Int J Pharm. 2017;518(1–2):138–54.
Li Q, Rudolph V, Weigl B, Earl A. Interparticle van der Waals force in powder flowability and compactibility. Int J Pharm. 2004;280(1–2):77–93.
Sun CC. Decoding powder tabletability: roles of particle adhesion and plasticity. J Adhes Sci Technol. 2011;25(4–5):483–99.
Zheng K, Lin Z, Capece M, Kunnath K, Chen L, Davé RN. Effect of particle size and polymer loading on dissolution behavior of amorphous griseofulvin powder. J Pharm Sci. 2019;108(1):234–42.
Monschke M, Kayser K, Wagner KG. Influence of particle size and drug load on amorphous solid dispersions containing pH-dependent soluble polymers and the weak base ketoconazole. AAPS PharmSciTech. 2021;22(1):1–1.
Chen H, Wang C, Kang H, Zhi B, Haynes CL, Aburub A, Sun CC. Microstructures and pharmaceutical properties of ferulic acid agglomerates prepared by different spherical crystallization methods. Int J Pharm. 2020;574:118914.
Schenck L, Mann AK, Liu Z, Milewski M, Zhang S, Ren J, Dewitt K, Hermans A, Cote A. Building a better particle: leveraging physicochemical understanding of amorphous solid dispersions and a hierarchical particle approach for improved delivery at high drug loadings. Int J Pharm. 2019;559:147–55.
Dahlberg C, Millqvist-Fureby A, Schuleit M. Surface composition and contact angle relationships for differently prepared solid dispersions. Eur J Pharm Biopharm. 2008;70(2):478–85.
Zhang S, Byrne G. Characterization of transport mechanisms for controlled release polymer membranes using focused ion beam scanning electron microscopy image-based modelling. J Drug Deliv Sci Technol. 2021;61:102136.
Zhang S, Wu D, Zhou L. Characterization of controlled release microspheres using FIB-SEM and image-based release prediction. AAPS PharmSciTech. 2020;21(5):194. https://doi.org/10.1208/s12249-020-01741-w.
Sun CC. Materials science tetrahedron-a useful tool for pharmaceutical research and development. J Pharm Sci. 2009;98(5):1671–87.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This study was internally funded by Genentech Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 268 kb)
Rights and permissions
About this article
Cite this article
Jia, W., Yawman, P., Pandya, K.M. et al. Assessing the Interrelationship of Microstructure, Properties, Drug Release Performance, and Preparation Process for Amorphous Solid Dispersions Via Noninvasive Imaging Analytics and Material Characterization. Pharm Res 39, 3137–3154 (2022). https://doi.org/10.1007/s11095-022-03308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03308-9