Abstract
Purpose
To explore the contribution of physiological characteristics to variability in ciclosporin pharmacokinetics in hematopoietic stem cell transplantation patients.
Methods
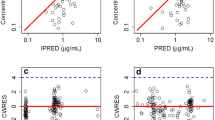
Clinical data from 563 patients were collected from centers in three regions. Ciclosporin concentrations were measured using immunoassays. The patients’ demographics, hematological and biological indicators, coadministered drugs, region, and disease diagnosis were recorded from medical records. Data analysis was performed using NONMEM based on a one-compartment model to describe the pharmacokinetics of ciclosporin. The reliability and stability of the final model were evaluated using bootstrap resampling, goodness-of-fit plots, and prediction-corrected visual predictive checks.
Results
The population estimate of the clearance (CL) was 30.4 L/h, the volume of distribution (V) was 874.0 L and the bioavailability (F) was 81.1%. The between-subject variability in these parameters was 26.3, 68.0, and 110.8%, respectively. Coadministration of fluconazole, itraconazole, or voriconazole decreased CL by 17.6%, 28.4%, and 29.2%, respectively. Females’ CL increased by approximately 12.0%. In addition, CL and V decreased with hematocrit, total protein, and uric acid increase, and CL also decreased with age and aspartate aminotransferase increase. However, CL increased with creatinine clearance increase.
Conclusions
A multicenter-based population pharmacokinetic model of ciclosporin was established. The pharmacokinetics of ciclosporin exhibited discrepancies among different regions.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
Abbreviations
- CL:
-
Clearance
- CMIA:
-
Chemiluminescent microparticle immunoassay
- CsA:
-
Ciclosporin
- ECLIA:
-
Electrochemiluminescence immunoassay
- EMIT:
-
Enzyme amplification immunoassay
- FFM:
-
Fat free mass
- FOCE:
-
First-order conditional estimation method
- FPIA:
-
Fluorescence polarization immunoassay
- HSCT:
-
Hematopoietic stem cell transplantation
- NFM:
-
Normal fat mass
- NONMEM:
-
Nonlinear mixed effect model
- OFV:
-
Objective function value
- pc_VPC:
-
Prediction-corrected visual predictive check
- Pop-PK:
-
Population pharmacokinetics
- PROP:
-
Proportional residual error
- RSE:
-
Relative standard error
- RUV:
-
Residual unidentified variability
- TCI:
-
Target concentration intervention
- TDM:
-
Therapeutic drug monitoring
- V:
-
Volume of distribution
References
McCune JS, Bemer MJ. Pharmacokinetics, pharmacodynamics and pharmacogenomics of Immunosuppressants in allogeneic Haematopoietic cell transplantation: part I. Clin Pharmacokinet. 2016;55(5):525–50.
Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61.
Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24(6):472–95.
Tafazoli A. Cyclosporine use in hematopoietic stem cell transplantation: pharmacokinetic approach. Immunotherapy. 2015;7(7):811–36.
Golubovic B, Prostran M, Miljkovic B, Vucicevic K, Radivojevic D, Grabnar I. Population pharmacokinetic approach of immunosuppressive therapy in kidney transplant patients. Curr Med Chem. 2016;23(19):1998–2011.
Minto C, Schnider T. Expanding clinical applications of population pharmacodynamic modelling. Br J Clin Pharmacol. 1998;46(4):321–33.
Yates CR, Zhang W, Song P, Li S, Gaber AO, Kotb M, et al. The effect of CYP3A5 and MDR1 polymorphic expression on cyclosporine oral disposition in renal transplant patients. J Clin Pharmacol. 2003;43(6):555–64.
Hesselink DA, van Gelder T, van Schaik RH, Balk AH, van der Heiden IP, van Dam T, et al. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin Pharmacol Ther. 2004;76(6):545–56.
Rousseau A, Leger F, Le Meur Y, Saint-Marcoux F, Paintaud G, Buchler M, et al. Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit. 2004;26(1):23–30.
Tokui K, Kimata T, Uchida K, Yuasa H, Hayashi Y, Itatsu T, et al. Dose adjustment strategy for oral microemulsion formulation of cyclosporine: population pharmacokinetics-based analysis in kidney transplant patients. Ther Drug Monit. 2004;26(3):287–94.
Wu KH, Cui YM, Guo JF, Zhou Y, Zhai SD, Cui FD, et al. Population pharmacokinetics of cyclosporine in clinical renal transplant patients. Drug metabolism and disposition: the biological fate of chemicals. 2005;33(9):1268–75.
Irtan S, Saint-Marcoux F, Rousseau A, Zhang D, Leroy V, Marquet P, et al. Population pharmacokinetics and bayesian estimator of cyclosporine in pediatric renal transplant patients. Ther Drug Monit. 2007;29(1):96–102.
Chen B, Zhang W, Gu Z, Li J, Zhang Y, Cai W. Population pharmacokinetic study of cyclosporine in Chinese renal transplant recipients. Eur J Clin Pharmacol. 2011;67(6):601–12.
Okada A, Ushigome H, Kanamori M, Morikochi A, Kasai H, Kosaka T, et al. Population pharmacokinetics of cyclosporine a in Japanese renal transplant patients: comprehensive analysis in a single center. Eur J Clin Pharmacol. 2017;73(9):1111–9.
Sun B, Li XY, Gao JW, Rui JZ, Guo YK, Peng ZH, et al. Population pharmacokinetic study of cyclosporine based on NONMEM in Chinese liver transplant recipients. Ther Drug Monit. 2010;32(6):715–22.
Rosenbaum SE, Baheti G, Trull AK, Akhlaghi F. Population pharmacokinetics of cyclosporine in cardiopulmonary transplant recipients. Ther Drug Monit. 2005;27(2):116–22.
Jacobson PA, Ng J, Green KG, Rogosheske J, Brundage R. Posttransplant day significantly influences pharmacokinetics of cyclosporine after hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9(5):304–11.
Eljebari H, Gaies E, Fradj NB, Jebabli N, Salouage I, Trabelsi S, et al. Population pharmacokinetics and Bayesian estimation of cyclosporine in a Tunisian population of hematopoietic stem cell transplant recipient. Eur J Clin Pharmacol. 2012;68(11):1517–24.
Wilhelm AJ, de Graaf P, Veldkamp AI, Janssen JJ, Huijgens PC, Swart EL. Population pharmacokinetics of ciclosporin in haematopoietic allogeneic stem cell transplantation with emphasis on limited sampling strategy. Br J Clin Pharmacol. 2012;73(4):553–63.
Zhou H, Gao Y, Cheng XL, Li ZD. Population pharmacokinetics of cyclosporine a based on NONMEM in Chinese allogeneic hematopoietic stem cell transplantation recipients. Eur J Drug Metab Pharmacokinet. 2012;37(4):271–8.
Zhou Y, Sheng XY, Xu JY, Bi SS, Lu W, Cui YM. Population pharmacokinetic study of cyclosporine in the hematopoietic stem cell transplant recipients. Int J Clin Pharmacol Ther. 2013;51(7):568–75.
Xue L, Zhang WW, Ding XL, Zhang JJ, Bao JA, Miao LY. Population pharmacokinetics and individualized dosage prediction of cyclosporine in allogeneic hematopoietic stem cell transplant patients. Am J Med Sci. 2014;348(6):448–54.
Kim MG, Kim IW, Choi B, Han N, Yun HY, Park S, et al. Population pharmacokinetics of cyclosporine in hematopoietic stem cell transplant patients: consideration of genetic polymorphisms. Ann Pharmacother. 2015;49(6):622–30.
Xiaoli D, Qiang F. Population pharmacokinetic study of cyclosporine in patients with nephrotic syndrome. J Clin Pharmacol. 2009;49(7):782–8.
Tsuji Y, Iwanaga N, Mizoguchi A, Sonemoto E, Hiraki Y, Ota Y, et al. Population pharmacokinetic approach to the use of low dose cyclosporine in patients with connective tissue diseases. Biol Pharm Bull. 2015;38(9):1265–71.
Mao JJ, Jiao Z, Yun HY, Zhao CY, Chen HC, Qiu XY, et al. External evaluation of population pharmacokinetic models for ciclosporin in adult renal transplant recipients. Br J Clin Pharmacol. 2018;84(1):153–71.
Wallemacq P, Maine GT, Berg K, Rosiere T, Marquet P, Aimo G, et al. Multisite analytical evaluation of the Abbott ARCHITECT cyclosporine assay. Ther Drug Monit. 2010;32(2):145–51.
Fung AWS, Knauer MJ, Blasutig IM, Colantonio DA, Kulasingam V. Evaluation of electrochemiluminescence immunoassays for immunosuppressive drugs on the Roche cobas e411 analyzer. F1000Res. 2017;6:1832.
Qin X, Rui J, Xia Y, Mu H, Song SH, Raja Aziddin RE, et al. Multi-center performance evaluations of Tacrolimus and cyclosporine Electrochemiluminescence immunoassays in the Asia-Pacific region. Ann Lab Med. 2018;38(2):85–94.
Robertshaw M, Lai KN, Swaminathan R. Prediction of creatinine clearance from plasma creatinine: comparison of five formulae. Br J Clin Pharmacol. 1989;28(3):275–80.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–52.
Germovsek E, Barker CIS, Sharland M, Standing JF. Pharmacokinetic-Pharmacodynamic modeling in pediatric drug development, and the importance of standardized scaling of clearance. Clin Pharmacokinet. 2019;58(1):39–52.
Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Gobburu JV, Lawrence J. Application of resampling techniques to estimate exact significance levels for covariate selection during nonlinear mixed effects model building: some inferences. Pharm Res. 2002;19(1):92–8.
Fanta S, Jonsson S, Backman JT, Karlsson MO, Hoppu K. Developmental pharmacokinetics of ciclosporin--a population pharmacokinetic study in paediatric renal transplant candidates. Br J Clin Pharmacol. 2007;64(6):772–84.
Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44(1):33–60.
Anderson BJ, Holford NH. Tips and traps analyzing pediatric PK data. Paediatr Anaesth. 2011;21(3):222–37.
Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-drug interactions. J NeuroImmune Pharmacol. 2006;1(3):323–39.
Akhlaghi F, Trull AK. Distribution of cyclosporin in organ transplant recipients. Clin Pharmacokinet. 2002;41(9):615–37.
Field KM, Dow C, Michael M. Part I: Liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9(11):1092–101.
Radcliffe NJ, Seah JM, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2017;8(1):6–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 4085 kb)
Rights and permissions
About this article
Cite this article
Xue, L., Zhang, Wj., Tian, Jx. et al. Multicenter-Based Population Pharmacokinetic Analysis of Ciclosporin in Hematopoietic Stem Cell Transplantation Patients. Pharm Res 37, 15 (2020). https://doi.org/10.1007/s11095-019-2740-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2740-2