Abstract
Purpose
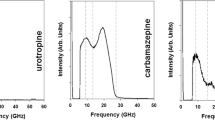
The unconventional tabletability of the indomethacin polymorphs – α and γ – are investigated from a topological and mechanical perspective using powder Brillouin light scattering (p-BLS) to identify the specific structure-performance relationship in these materials.
Method
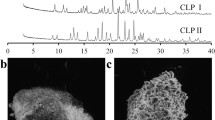
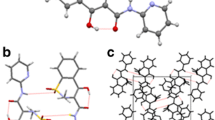
Indomethacin (γ-form) was purchased and used to prepare the α polymorph. Powder X-ray diffraction was used to confirm phase identity, while p-BLS was used to obtain the mechanical properties. Energy frameworks were determined with Crystal Explorer to visualize the interaction topologies. Using a Carver press and a stress-strain analyzer, the tableting performance of each polymorph was determined.
Results
Polymorph-specific acoustic frequency distributions were observed with distinct, zero-porosity, aggregate elastic moduli determined. The p-BLS spectra for α-indomethacin display a population of low-velocity shear modes, indicating a direction of facilitated shear. This improves slip-mediated plasticity and tabletability. Our p-BLS spectra experimentally indicates that a low-energy slip system is available to α-indomethacin which supports ours and previous energy framework calculations. Despite a 2d-layered crystal motif favorable for shear deformation, the γ-form displays a higher shear modulus that is supported by our hydrogen-bonding analysis of γ-indomethacin.
Conclusion
Our experimental, mechanical data is consistent with the predicted interaction topologies and these two inputs combined permit a comprehensive, molecular understanding of polymorph-specific tabletability.







Similar content being viewed by others
References
Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery – a review. Pharmaceutical Science & Technology Today. 2000;3(4):138–45.
Sun W-J, Aburub A, Sun CC. Particle engineering for enabling a formulation platform suitable for manufacturing low-dose tablets by direct compression. J Pharm Sci. 2017;106:1772–7.
Chen X, Wen H, Park K. Challenges and new Technologies of Oral Controlled Release. In: Wen H, editor. Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice John Wiley & Sons, Inc.; 2010. p. 257–77.
Newman AW, Byrn SR. Solid-state analysis of the active pharmaceutical ingredient in drug products. Drug Discov Today. 2003;8(19):898–905.
Bag PP, Chen M, Sun CC, Reddy CM. Direct correlation among crystal structure, mechanical behaviour and Tabletability in a Trimorphic molecular compound. Cryst Eng Comm. 2012;14:3865–7.
Aitipamula S, Vangala VR. X-ray crystallography and its role in understanding the physicochemical properties of pharmaceutical Cocrystals. Journal of the Indian Institute of Science. 2017;97(2):227–43.
Kawashima Y, Imai M, Takeuchi H, Yamamoto H, Kamiya K, Hino T. Improved Flowability and Compactability of spherically agglomerated crystals of ascorbic acid for direct tableting designed by spherical crystallization process. Powder Technol. 2003;130(1–3):283–9.
Kamahara T, Takasuga M, Tung HH, Hanaki K, Fukunaka T, Izzo B, et al. Generation of fine pharmaceutical particles via controlled secondary nucleation under high shear environment during crystallization − process development and scale-up. Org Process Res Dev. 2007;11(4):699–703.
Chang S-Y, Sun CC. Superior plasticity and Tabletability of theophylline monohydrate. Mol Pharm. 2017;14:2047–55.
Desiraju GR. Crystal Engineering: From Molecule to Crystal J Am Chem Soc. 2013;135:9952–67.
Varughese S, Kiran MSRN, Solanko KA, Bond AD, Ramamurty U, Desiraju GR. Interaction anisotropy and shear instability of Asprin polymorphs established by Nanoindentation. Chem Sci. 2011;2(11):2236–42.
Ghosh S, Reddy CM. Elastic and bendable caffeine Cocrystals: implications for the Design of Flexible Organic Materials. Angew Chem Int Ed. 2012;51(41):10319–23.
Mishra MK, Varughese S, Ramamurty U, Desiraju GR. Odd–Even Effect in the Elastic Modulii of α,ω-Alkanedicarboxylic Acids. J Am Chem Soc. 2013;135(22):8121–4.
Nangia A. Supramolecular Chemistry and Crystal Engineering. J Chem Sci. 2010;122(3):295–310.
Desiraju GR. Crystal engineering: structure, property and beyond. I U Cr J. 2017;4(6):710–1.
Anbalagan P, Liew CV, Heng PWS. Role of dwell on compaction deformation during tableting: an overview. Journal of Pharmaceutical Investigation. 2017;47(3):179–81.
Wang C, Sun CC. Identifying Slip Planes in Organic Polymorphs by Combined Energy Framework Calculations and Topology Analysis Cryst Growth Des. 2018;18:1909–16.
Singaraju AB, Iyer M, Haware RV, Stevens LL. Caffeine Co-Crystal Mechanics Evaluated with a Combined Structural and Spectroscopic Approach Cryst Growth Des 2016;16:4383–4391.
Ahmed H, Shimpi MR, Velaga SP. Relationship between mechanical properties and crystal structure in cocrystals and salt of paracetamol. Drug Dev Ind Pharm. 2017;43(1):89–97.
Guiry KP, Kelleher JM, Lawrence SE, Mcauliffe MT, Moynihan HA, Ryan AL. Crystal polymorphism of pharmaceuticals: probing crystal nucleation at the molecular level. J Enzyme Inhib Med Chem 2007;22(5):550–5, 555.
Persson A-S, Ahmed H, Velaga S, Alderborn Go. Powder Compression Properties of Paracetamol, Paracetamol Hydrochloride, and Paracetamol Cocrystals and Coformers. J Pharm Sci. 2018;107:1920–7.
Singaraju AB, Nguyen K, Jain A, Haware RV, Stevens LL. Aggregate Elasticity, Crystal Structure, and Tableting Performance for p-Aminobenzoic Acid and a Series of Its Benzoate Esters. Mol Pharmaceutics. 2016;13:3794–806.
Sun C, Grant DJ. Influence of crystal structure on the tableting properties of Sulfamerazine polymorphs. Pharm Res. 2001;18(3):274–80.
Khomane KS, More PK, Raghavendra G, Bansal AK. Molecular understanding of the compaction behavior of indomethacin polymorphs. Mol Pharm. 2013;10(2):631–9.
Singaraju AB, Bahl D, Stevens LL. Brillouin light scattering: development of a near century-old technique for characterizing the mechanical properties of materials. AAPS PharmSciTech. 2019;20(3):109.
Singaraju AB, Nguyen K, Gawedzki P, Herald F, Meyer G, Wentworth D, et al. Combining crystal structure and interaction topology for interpreting functional molecular solids: A study of theophylline Cocrystals. Cryst Growth Des. 2017;17(12):6741–51.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The Cambridge structural database. Acta Crystallographica Section B. 2016;72(2):171–9.
Hernandez J, Li G, Cummins HZ, Callender RH. Low-frequency light-scattering spectroscopy of powders. J Opt Soc Am B. 1996;13(6):1130–4.
Bahl D, Singaraju AB, Stevens LL. Aggregate elasticity and Tabletability of molecular solids: a validation and application of powder Brillouin light scattering. Journal of the American Association of Pharmaceutical Scientists. 2018;19(8):3430–9.
Juban A, Briançon S, Puel F, Hoc T, Nouguier-Lehon C. Experimental study of tensile strength of pharmaceutical tablets: effect of the diluent nature and compression pressure. EPJ Web Conf. 2017;140:13002.
Turner MJ, Grabowsky S, Jaytilaka D, Spackman MA. Accurate and efficient model energies for exploring intermolecular interactions in molecular crystals. J Phys Chem Lett. 2014;5(24):4249–55.
Wolff SK, Grimwood DJ. McKinnon JJ. Turner MJ: Jayatilaka D, Spackman MA. University of Western Australia; 2012.
Akande OF, Ford JL, Rowe PH, Rubinstein MH. Pharmaceutics: the effects of lag-time and dwell-time on the compaction properties of 1:1 paracetamol/microcrystalline cellulose tablets prepared by pre-compression and Main compression. J Pharm Pharmacol. 1998;50(1):19–28.
Patel S, Kaushal AM, Bansal AK. Compression Physics in the Formulation Development of Tablets. Ther Drug Carrier Syst. 2006;23(1):1–65.
Shibata T, Kojima S. Liquid, glass, and crystalline indomethacin studied by Brillouin scattering. Japanese Jouranl of Applied Physics. 2018;57(7L1):07LB3.
Osei-Yeboah F, Chang S-Y, Sun CC. A critical Examination of the Phenomenon of Bonding Area - Bonding Strength Interplay in Powder Tableting. Pharm Res. 2016;33(5):1126–32.
Eriksson M, Alderborn G. The effect of particle fragmentation and deformation on the Interparticulate Bond formation process during powder compaction. Pharm Res. 1995;12:1031–9.
Nordström J, Klevan I, Alderborn G. A protocol for the classification of powder compression characteristics. Eur J Pharm Biopharm. 2012;80:209–16.
Lamešić D, Planinšek O, GermanIlić I. Modified equation for particle bonding area and strength with inclusion of powder fragmentation propensity. Eur J Pharm Sci. 2018;121:218–27.
Shishkin OV, Dyakonenko VV, Maleev AV. Supramolecular architecture of crystals of fused hydrocarbons based on topology of intermolecular interactions. Cryst Eng Comm. 2012;14:1795–804.
Cyrański MK, Jamróz MH, Rygula A, Dobrowolski JC, Dobrzycki Ł, Baranska M. On two alizarin polymorphs. CrystEngComm. 2012;14(10):3667–76.
Sun CC, Kiang YH. On the identification of slip Planes in organic crystals based on attachment energy calculation. J Pharm Sic. 2007;97(8):3456–61.
Acknowledgments and Disclosures
B.A.Y. was supported by the Terrence and Donna Dahl Pharmaceutics Fellowship during the course of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 339 kb)
Rights and permissions
About this article
Cite this article
Young, B.A., Bahl, D. & Stevens, L.L. Understanding the Tabletability Differences between Indomethacin Polymorphs Using Powder Brillouin Light Scattering. Pharm Res 36, 150 (2019). https://doi.org/10.1007/s11095-019-2681-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2681-9