Abstract
Purpose
This work focused on the preparation of polycaprolactone based nanoparticles containing indomethacin to provide topical analgesic and anti-inflammatory effect for symptomatic treatment of inflammatory diseases. Indomethacin loaded nanoparticles are prepared for topical application to decrease indomethacin side effects and administration frequency. Oppositely to already reported works, in this research non-invasive method has been used for the enhancement of indomethacin dermal drug penetration. Ex-vivo skin penetration study was carried out on fresh human skin.
Methods
Nanoprecipitation was used to prepare nanoparticles. Nanoparticles were characterized using numerous techniques; dynamic light scattering, SEM, TEM, DSC and FTIR. Regarding ex-vivo skin penetration of nanoparticles, confocal laser scanning microscopy has been used.
Results
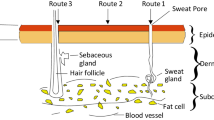
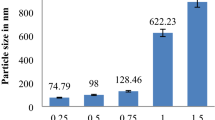
The results showed that NPs hydrodynamic size was between 220 to 245 nm and the zeta potential value ranges from −19 to −13 mV at pH 5 and 1 mM NaCl. The encapsulation efficiency was around 70% and the drug loading was about 14 to 17%. SEM and TEM images confirmed that the obtained nanoparticles were spherical with smooth surface. The prepared nanoparticles dispersions were stable for a period of 30 days under three temperatures of 4°C, 25°C and 40°C. In addition, CLSM images proved that obtained NPs can penetrate the skin as well.
Conclusion
The prepared nanoparticles are submicron in nature, with good colloidal stability and penetrate the stratum corneum layer of the skin. This formulation potentiates IND skin penetration and as a promising strategy would be able to decline the side effects of IND.









Similar content being viewed by others
Abbreviations
- COX enzyme:
-
Cyclooxygenase enzyme
- CLSM:
-
Confocal laser scanning microscopy
- DSC:
-
Differential scanning calorimeter
- EE:
-
Encapsulation efficiency
- FTIR:
-
Fourier transform infrared spectroscopy
- IND:
-
Indomethacin
- NPs:
-
Nanoparticles
- NSAIDs:
-
Non-Steroidal Anti-inflammatory Drugs
- PCL:
-
Polycaprolactone
- PVA:
-
Polyvinyl alcohol
- RT:
-
Room temperature (25°C)
- SEM:
-
Scanning Electron Microscopy
- TEM:
-
Transmission Electron Microscopy
References
Cordero JA, Camacho M, Obach R, Domenech J, Vila L. In vitro based index of topical anti-inflammatory activity to compare a series of NSAIDs. Eur J Pharm Biopharm. 2001;51:135–42.
Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24:121–32.
Bateman DN. Non-steroidal anti-inflammatory drugs. Medicine (Baltimore). 2012;40:140.
Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–33.
Ziltener J-L, Leal S, Fournier P-E. Non-steroidal anti-inflammatory drugs for athletes: an update. Ann Phys Rehabil Med. 2010;53:278–82. 282–8
Závišová V, Koneracká M, Štrbák O, Tomašovičová N, Kopčanský P, Timko M, et al. Encapsulation of indomethacin in magnetic biodegradable polymer nanoparticles. J Magn Magn Mater. 2007;311:379–82.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8.
Tomoda K, Terashima H, Suzuki K, Inagi T, Terada H, Makino K. Enhanced transdermal delivery of indomethacin using combination of PLGA nanoparticles and iontophoresis in vivo. Colloids Surf B: Biointerfaces. 2012;92:50–4.
Suksiriworapong J, Sripha K, Kreuter J, Junyaprasert VB. Comparative study of Ibuprofen and Indomethacin loaded poly (caprolactone) nanoparticles: physicochemical properties. J Magn Magn Mater. 2010;37:17–27.
Iqbal M, Zafar N, Fessi H, Elaissari A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm. 2015;496:173–90.
Jelvehgari M, Montazam SH. Comparison of Microencapsulation by Emulsion-Solvent Extraction/Evaporation Technique Using Derivatives Cellulose and Acrylate-Methacrylate Copolymer as Carriers. Jundishapur J Nat Pharm Prod. 2012;7:144–52.
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–4.
Miladi K, Sfar S, Fessi H, Elaissari A. Nanoprecipitation process: from particle preparation to in vivo applications. Polymer Nanoparticles for Nanomedicines 2016. p. 17–53.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–42.
Park J-H, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release Off J Control Release Soc. 2005;104:51–66.
Dash TK, Konkimalla VB. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J Control Release Off. 2012;158:15–33.
Badri W, Miladi K, Nazari QA, Fessi H, Elaissari A. Effect of process and formulation parameters on polycaprolactone nanoparticles prepared by solvent displacement. Colloids Surf A Physicochem Eng Asp. 2017;516:238–44.
Pygall SR, Whetstone J, Timmins P, Melia CD. Pharmaceutical applications of confocal laser scanning microscopy: The physical characterisation of pharmaceutical systems. Adv Drug Deliv Rev. 2007;59:1434–52.
Alvarez-Román R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release. 2004;99:53–62.
Zhang X, Zhang X, Wang S, Liu M, Tao L, Wei Y. Surfactant modification of aggregation-induced emission material as biocompatible nanoparticles: Facile preparation and cell imaging. Nanoscale. 2012;5:147–50.
Adibkia K, Javadzadeh Y, Dastmalchi S, Mohammadi G, Niri FK, Alaei-Beirami M. Naproxen-eudragit RS100 nanoparticles: preparation and physicochemical characterization. Colloids Surf B: Biointerfaces. 2011;83:155–9.
Gratieri T, Schaefer UF, Jing L, Gao M, Kostka K-H, Lopez RFV, et al. Penetration of quantum dot particles through human skin. J Biomed Nanotechnol. 2010;6:586–95.
Vulovic N, Primorac M, Stupar M, Ford JL. Some studies into the properties of indomethacin suspensions intended for ophthalmic use. Int J Pharm. 1989;55:123–8.
Gupta KC, Haider A, Choi Y, Kang I. Nanofibrous scaffolds in biomedical applications. Biomater Res. 2014;18:5.
Braz WR, Rocha NL, de Faria EH, Silva MLAE, Ciuffi KJ, Tavares DC, et al. Incorporation of anti-inflammatory agent into mesoporous silica. Nanotechnology. 2016;27(38):385,103.
Elzein T, Nasser-Eddine M, Delaite C, Bistac S, Dumas P. FTIR study of polycaprolactone chain organization at interfaces. J Colloid Interface Sci. 2004;273:381–7.
Lin H-L, Zhang G-C, Lin S-Y. Real-time co-crystal screening and formation between indomethacin and saccharin via DSC analytical technique or DSC–FTIR microspectroscopy. J Therm Anal Calorim. 2015;120:679–87.
Tomoda K, Terashima H, Suzuki K, Inagi T, Terada H, Makino K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf B: Biointerfaces. 2011;88:706–10.
Acknowledgments and Disclosures
Waisudin Badri gratefully acknowledges French foreign affairs ministry and Afghanistan higher education ministry for providing the PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badri, W., Miladi, K., Robin, S. et al. Polycaprolactone Based Nanoparticles Loaded with Indomethacin for Anti-Inflammatory Therapy: From Preparation to Ex Vivo Study. Pharm Res 34, 1773–1783 (2017). https://doi.org/10.1007/s11095-017-2166-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2166-7